Abstract
OBJECTIVE
Incorporation of comorbidity burden to inform diabetes management in older adults remains challenging. High-sensitivity cardiac troponins are objective, quantifiable biomarkers that may improve risk monitoring in older adults. We assessed the associations of elevations in high-sensitivity cardiac troponin I (hs-cTnI) and T (hs-cTnT) with comorbidities and improvements in mortality risk stratification.
RESEARCH DESIGN AND METHODS
We used logistic regression to examine associations of comorbidities with elevations in either troponin (≥85th percentile) among 1,835 participants in the Atherosclerosis Risk in Communities (ARIC) Study with diabetes (ages 67–89 years, 43% male, 31% black) at visit 5 (2011–2013). We used Cox models to compare associations of high cardiac troponins with mortality across comorbidity levels.
RESULTS
Elevations in either troponin (≥9.4 ng/L for hs-cTnI, ≥25 ng/L for hs-cTnT) were associated with prevalent coronary heart disease, heart failure, chronic kidney disease, pulmonary disease, hypoglycemia, hypertension, dementia, and frailty. Over a median follow-up of 6.2 years (418 deaths), both high hs-cTnI and high hs-cTnT further stratified mortality risk beyond comorbidity levels; those with a high hs-cTnI or hs-cTnT and high comorbidity were at highest mortality risk. Even among those with low comorbidity, a high hs-cTnI (hazard ratio 3.0 [95% CI 1.7, 5.4]) or hs-cTnT (hazard ratio 3.3 [95% CI 1.8, 6.2]) was associated with elevated mortality.
CONCLUSIONS
Many comorbidities were reflected by both hs-cTnI and hs-cTnT; elevations in either of the troponins were associated with higher mortality risk beyond comorbidity burden. High-sensitivity cardiac troponins may identify older adults at high mortality risk and be useful in guiding clinical care of older adults with diabetes.
Introduction
Guidelines from several professional societies, including the American Diabetes Association, endorse the individualization of treatment goals on the basis of comorbidity and functional status of the older patient with diabetes (1–4). However, there is a lack of clarity on how to operationalize this information (5). One approach presented by the American Diabetes Association suggests using a count of comorbidities on the basis of a prespecified list to inform management of hyperglycemia in older adults (6), with a high burden of multiple comorbidities defined as having three or more co-occurring conditions, which include arthritis, cancer, congestive heart failure, depression, emphysema, falls, hypertension, incontinence, stage 3 or worse chronic kidney disease, myocardial infarction, and stroke (7). High comorbidity burden is associated with mortality risk (8) and diminished benefits from stringent glycemic control, which are typically realized over a relatively long time frame (9). Despite guideline recommendations to account for comorbidity burden in older age, objective approaches are lacking; it remains unclear which comorbidities to consider (10–12) and how to quantify overall burden or use this information specifically to guide treatment. Furthermore, reliance on clinical comorbidities ignores the substantial burden of subclinical disease in older adults (13).
Among older adults with diabetes, cardiovascular disease is the leading cause of death (14,15). Cardiac troponins have served as the cornerstone for the diagnosis of acute myocardial infarction for decades (16). However, new high-sensitivity assays are able to detect circulating concentrations of high-sensitivity cardiac troponins I (hs-cTnI) and T (hs-cTnT) in 50–100% of the general ambulatory population (17). These concentrations of hs-cTnI and hs-cTnT are far below the diagnostic threshold for an acute coronary event and are indicators of subclinical myocardial damage. Prior studies have demonstrated that hs-cTnI and hs-cTnT are potent predictors of cardiovascular morbidity and mortality risk beyond traditional cardiovascular risk factors in population-based studies (18–22). Among middle-aged adults, the two troponins are only moderately correlated with each other, and their correlates differ, with diabetes being more strongly associated with hs-cTnT than hs-cTnI (23). There is growing evidence in ambulatory populations that hs-cTnI and hs-cTnT are complementary, rather than interchangeable, markers of cardiovascular disease and mortality risk (21,22). Both biomarkers are higher in older age (24,25) and may reflect the cumulative impact of various comorbidities on cardiovascular health (26). Whether hs-cTnI and hs-cTnT could serve as objective markers of overall health in older adults remains uncharacterized.
We hypothesized that hs-cTnI and hs-cTnT may either replace or supplement comorbidity burden in the prediction of mortality risk in older adults with diabetes. Therefore, we assessed 1) whether the comorbidities highlighted in the American Diabetes Association guidelines and geriatric syndromes (dementia and frailty) were associated with hs-cTnI and hs-cTnT and 2) whether hs-cTnI and hs-cTnT could be used to improve stratification of mortality risk beyond traditional risk factors and comorbidity burden in older adults with diabetes.
Research Design and Methods
Study Population
We included 1,835 older adults with diabetes (ages 67–89 years) in the Atherosclerosis Risk in Communities (ARIC) Study who attended visit 5 (2011–2013). We excluded participants according to standard ARIC race-center exclusions (self-reported race other than black or white or low enrollment of blacks or whites at certain study sites) or who had missing covariate data (Supplementary Fig. 1). Diabetes was defined as a self-reported prior physician diagnosis, diabetes medication use, or a hemoglobin A1c ≥6.5%. Details on the ARIC Study design and data collection have been previously published (27). All ARIC protocols were approved by institutional review boards at each study site, and all participants provided written informed consent.
Comorbidities
All comorbidities were assessed at visit 5 (2011–2013). History of coronary heart disease or stroke was assessed on the basis of self-report at ARIC visit 1 (1987–1989) or a subsequent adjudicated hospitalization diagnosis between visits 1 and 5. History of heart failure was based on self-report at ARIC visit 1 or subsequent hospitalization before visit 5. Cancer history was ascertained through linkage to cancer registries and prospective follow-up since 2011 (28). Depression was assessed on the basis of a Center for Epidemiological Studies Depression Scale (29) screening score of ≥16 or self-reported depression affecting daily life. Emphysema or chronic obstructive pulmonary disease was assessed through self-report at visit 5. History of falls was determined using hospitalization ICD-9 codes before visit 5. History of severe hypoglycemia was ascertained from hospitalization ICD-9 codes in the primary position on the basis of records from ARIC surveillance of local community hospitals and medical record abstractions for hospitalizations outside the ARIC surveillance system (30). Additional events were identified with linkage to Medicare claims for hospitalizations, emergency department visits, and ambulance services available for participants enrolled in Medicare fee-for-service Part B (31,32). Arthritis status was ascertained on the basis of self-report at visit 4 (1996–1998) and updated on the basis of hospitalization or Medicare claims (ICD-9 codes 714–715). Hypertension was defined as a mean blood pressure ≥140/90 mmHg or prior diagnosis or hypertension medication use. Incontinence was defined on the basis of hospitalization or Medicare claims (ICD-9 code 788.3) before visit 5. Stage 3+ chronic kidney disease was defined as an estimated glomerular filtration rate <60 mL/min/1.73 m2 as calculated by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation using cystatin C and creatinine (33). High comorbidity burden was defined according to the American Diabetes Association recommendations of three or more of the above comorbidities (each comorbidity was weighted equally). Dementia status was assessed on the basis of detailed neurocognitive testing and adjudication (34). Frailty was defined on the basis of Fried et al. (35) criteria as previously described in ARIC (36).
Biomarkers of Cardiomyocyte Injury
Level of hs-cTnI was measured in stored plasma using the Architect STAT Troponin-I assay (Abbott Diagnostics, Chicago, IL). Values below the level of blank (1.2 ng/L) were imputed to 0.6 ng/L. Level of hs-cTnT was measured in stored plasma using the Elecsys Troponin T Gen 5 STAT assay (Roche Diagnostics, Indianapolis, IN). Values below the level of blank (3 ng/L) were imputed to 1.5 ng/L. While there is higher imprecision of measurements between the level of blank and level of detection of these assays, we censored at the level of blank because prior work has demonstrated population-level prognostic signal at the very low concentrations between the level of blank and level of detection (37).
Outcome Ascertainment
Active surveillance for all-cause mortality was based on linkage to the National Death Index and semiannual telephone calls to participants and/or their surrogates. The last date of follow-up was 31 December 2018.
Statistical Analyses
In analyses of the independent associations of hs-cTnI and hs-cTnT with mortality risk, we categorized each troponin on the basis of equipercentile groups starting at the 40th percentile (<40th, 40th to <55th, 55th to <70th, 70th to <85th, and ≥85th percentile). Because there are no predefined clinical thresholds for hs-cTnI and hs-cTnT for risk prediction among the general population, we defined high troponin as concentrations ≥85th percentile in the ARIC population at visit 5 (≥24 ng/L for hs-cTnT, ≥9.2 ng/L for hs-cTnI) corresponding to the highest percentile category. We used logistic regression to assess the associations of each comorbidity with elevations in either hs-cTnT or hs-cTnI, with adjustment for age, sex, and race-center. To estimate the sensitivity, specificity, likelihood ratios, and positive and negative predictive values for hs-cTnI and hs-cTnT cut points at the 90th, 95th, and 99th percentiles in the cohort, we considered mortality as a dichotomous outcome.
We used Cox proportional hazards to model the association of cardiac troponins and comorbidity burden (high [three or more] vs. low [fewer than three]) with all-cause mortality with adjustment for age, male sex, race-center, current smoking, systolic blood pressure, diastolic blood pressure, antihypertensive medication use, total cholesterol, HDL cholesterol, cholesterol-lowering medication use, and hemoglobin A1c. We assessed the interactions of each association with sex. To compare survival models with and without hs-cTnI or hs-cTnT, we calculated the change in C-statistics (38) and the proportions reclassified either at higher or at lower risk among those with and without the outcome. A base model included all covariates. The base model was compared with models that added high comorbidity, high hs-cTnI, continuous hs-cTnI, high hs-cTnT, or continuous hs-cTnT. Models with troponin measures were similarly compared with a base model that included high comorbidity. We also calculated the weighted net reclassification index, which takes into account the prevalence of the outcome in weighting the relative event and nonevent net reclassification indexes (39). The 95% CIs for the percent reclassified were calculated using bootstrapping with 1,000 iterations. To assess the association of each comorbidity with mortality, we used Cox models adjusted for age, sex, race-center (model 1), current smoking, systolic blood pressure, diastolic blood pressure, antihypertensive medication use, total cholesterol, HDL cholesterol, cholesterol-lowering medication use, and hemoglobin A1c (model 2). We further adjusted for hs-cTnI (model 3a), hs-cTnT (model 3b), or both (model 3c). In these models, each troponin was modeled using log2-transformation with five equipercentile linear spline knots. Statistical significance was set at a two-sided α of 0.05.
Results
Participants were 43% male and 31% black with a mean (SD) age of 75.5 (5.1) years. By American Diabetes Association definitions, 61% of the participants had high comorbidity burden. Those with a higher burden were more likely to be older, have higher hs-cTnI and hs-cTnT, and have a slightly higher hemoglobin A1c (Table 1). Those with higher circulating troponin had higher average blood pressure, more comorbidities, and slightly higher hemoglobin A1c (Supplementary Tables 1 and 2).
Table 1.
Baseline characteristics of older adults with diabetes according to comorbidity burden: the ARIC Study, visit 5 (2011–2013), N = 1,853
| Low comorbidity burden(<3 comorbidities) | High comorbidity burden(≥3 comorbidities) | |
|---|---|---|
| n | 723 | 1,130 |
| Age (years), mean (SD) | 73.8 (4.4) | 76.6 (5.2) |
| Male, % | 45.0 | 41.5 |
| Black race, % | 31.8 | 29.8 |
| Current smoker, % | 5.7 | 5.8 |
| BMI* (kg/m2), mean (SD) | 30.1 (5.4) | 31.2 (6.2) |
| Blood pressure (mmHg), mean (SD) | ||
| Systolic | 129.3 (16.9) | 131.0 (20.0) |
| Diastolic | 66.1 (9.7) | 64.2 (11.4) |
| Hypertension medication use, % | 67.9 | 90.2 |
| Total cholesterol (mmol/L), mean (SD) | 4.4 (1.0) | 4.3 (1.1) |
| HDL cholesterol (mmol/L), mean (SD) | 1.3 (0.3) | 1.2 (0.3) |
| Cholesterol-lowering medication use, % | 63.2 | 74.4 |
| American Diabetes Association comorbidities, % | ||
| Coronary heart disease | 3.7 | 29.7 |
| Heart failure | 2.9 | 36.2 |
| Stroke | 1.5 | 7.7 |
| Arthritis | 48.7 | 86.4 |
| Cancer | 9.3 | 32.7 |
| Chronic kidney disease, stage 3+ | 14.4 | 65.0 |
| Depression | 0.4 | 5.0 |
| Emphysema or COPD | 1.8 | 11.3 |
| History of hospitalized fall | 0.6 | 5.7 |
| History of severe hypoglycemia | 0.4 | 4.7 |
| Hypertension | 72.3 | 93.4 |
| Incontinence | 1.7 | 19.6 |
| Geriatric syndromes, % | ||
| Dementia | 2.9 | 6.4 |
| Frailty | 4.8 | 13.9 |
| hs-cTnI (ng/L), median (Q1, Q3) | 2.8 (1.9, 4.5) | 4.3 (2.8, 7.7) |
| hs-cTnT (ng/L), median (Q1, Q3) | 10.0 (7.0, 14.0) | 14.0 (9.0, 22.0) |
| Hemoglobin A1c (%), mean (SD) | 6.5 (1.0) | 6.7 (1.1) |
| Diabetes medication use, % | ||
| No medication | 45.8 | 39.1 |
| Oral only | 43.2 | 43.1 |
| Insulin only | 4.7 | 8.8 |
| Insulin and oral | 6.4 | 9.0 |
COPD, chronic obstructive pulmonary disease; Q, quartile.
n = 20 missing values for BMI (4 with low comorbidity burden, 16 with high comorbidity burden).
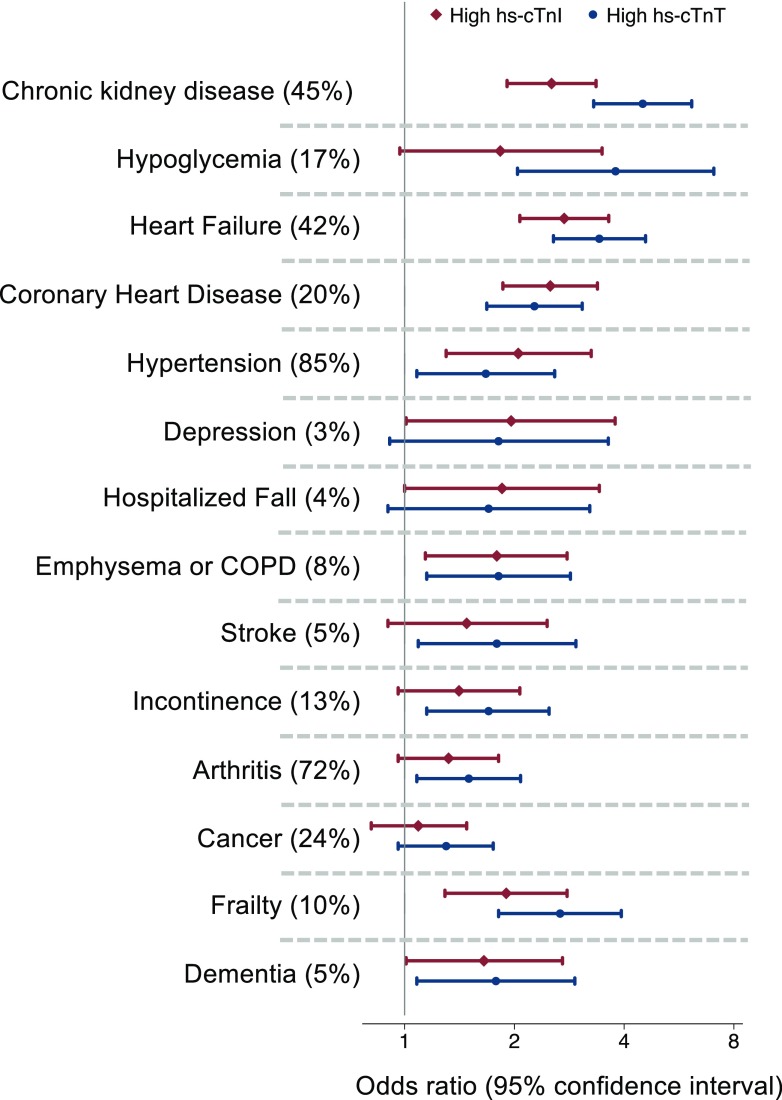
Both hs-cTnI and hs-cTnT reflected similar comorbidity profiles. High hs-cTnI and hs-cTnT were both associated with coronary heart disease, heart failure, chronic kidney disease, emphysema or chronic obstructive pulmonary disease, and hypertension. Both troponins were associated with frailty and dementia (Fig. 1).
Figure 1.
Age-, sex-, and race-center–adjusted odds ratios (95% CIs) of the association of each comorbidity (prevalence) with high hs-cTnI (≥85th percentile) or high hs-cTnT (≥85th percentile). COPD, chronic obstructive pulmonary disease.
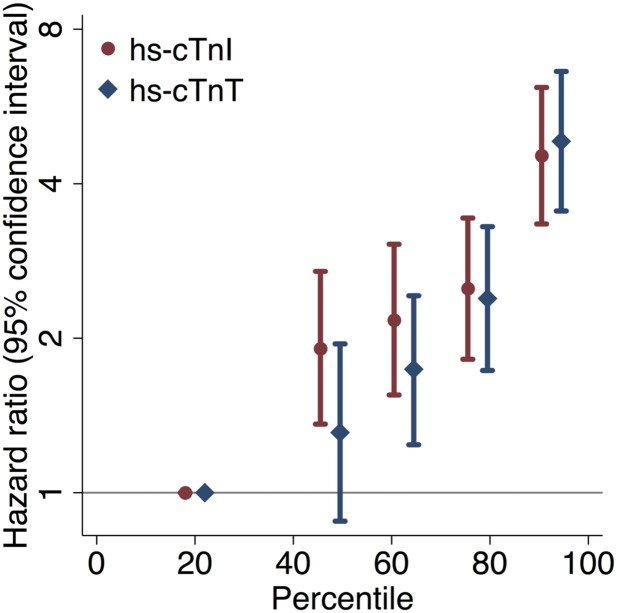
Over a median follow-up of 6.2 years (maximum 7.6 years), there were 418 deaths. Higher levels of hs-cTnI and hs-cTnT were associated with higher all-cause mortality risk in the full cohort (Fig. 2) and among those without a history of cardiovascular disease (Supplementary Fig. 2). The 4-year cumulative mortality among those with troponin values <40th percentile was 5.4% (hs-cTnI) and 5.4% (hs-cTnT) compared with 27.8% (hs-cTnI) and 29.4% (hs-cTnT) among those with hs-cTnI or hs-cTnT ≥85th percentile. Considering mortality status dichotomously over follow-up, cut points of hs-cTnI and hs-cTnT at the 90th, 95th, and 99th percentiles were associated with high specificity (Supplementary Table 3).
Figure 2.
Hazard ratios (95% CIs) of the association of hs-cTnI and hs-cTnT percentile categories with all-cause mortality, adjusted for age, male sex, race-center, current smoking, systolic blood pressure, diastolic blood pressure, antihypertensive medication use, total cholesterol, HDL cholesterol, cholesterol-lowering medication use, and hemoglobin A1c.
High comorbidity burden (three or more comorbidities) was associated with elevated mortality risk (hazard ratio 2.94 [95% CI 2.25, 3.85]) compared with those with a low burden (fewer than three comorbidities) and was associated with improvements in the C-statistic of predictive models for mortality (Table 2). Both dichotomized and continuous hs-cTnI and hs-cTnT modestly improved the C-statistics associated with mortality models compared with the base model. C-statistics associated with models containing either log2-transformed hs-cTnI or hs-cTnT and traditional risk factors were comparable to models containing traditional risk factors and comorbidity burden (Table 2). The inclusion of hs-cTnI and hs-cTnT predominantly downclassified those who did not die during follow-up (Supplementary Table 4).
Table 2.
C-statistics of models of all-cause mortality with the addition of comorbidity burden, hs-cTnI, or hs-cTnT compared with a base model of cardiovascular risk factors
| C-statistic (95% CI) | ΔC-statistic (95% CI) | P value | |
|---|---|---|---|
| Base model* | 0.6662 (0.6386, 0.6938) | — | — |
| Comorbidity burden (≥3 comorbidities) | 0.7024 (0.6781, 0.7267) | 0.0362 (0.0194, 0.0530)† | — |
| hs-cTnI ≥85th percentile | 0.6862 (0.6586, 0.7138) | 0.0200 (0.0067, 0.0333)† | 0.09‡ |
| Log2(hs-cTnI) | 0.7030 (0.6766, 0.7295) | 0.0369 (0.0223, 0.0514)† | 0.94‡ |
| hs-cTnT ≥85th percentile | 0.6951 (0.6677, 0.7226)a | 0.0289 (0.0143, 0.0436)† | 0.45‡ |
| Log2(hs-cTnT) | 0.7172 (0.6912, 0.7432)b | 0.0510 (0.0318, 0.0703)† | 0.15‡ |
Age, male sex, race-center, current smoking, systolic blood pressure, diastolic blood pressure, antihypertensive medication use, total cholesterol, HDL cholesterol, cholesterol-lowering medication use, and hemoglobin A1c.
P < 0.01.
Compared with base model + comorbidity burden (≥3 comorbidities).
P = 0.20 compared with base model with comorbidity burden (≥3 comorbidities) + hs-cTnI ≥85th percentile.
P = 0.09 compared with base model with comorbidity burden (≥3 comorbidities) + log2(hs-cTnI).
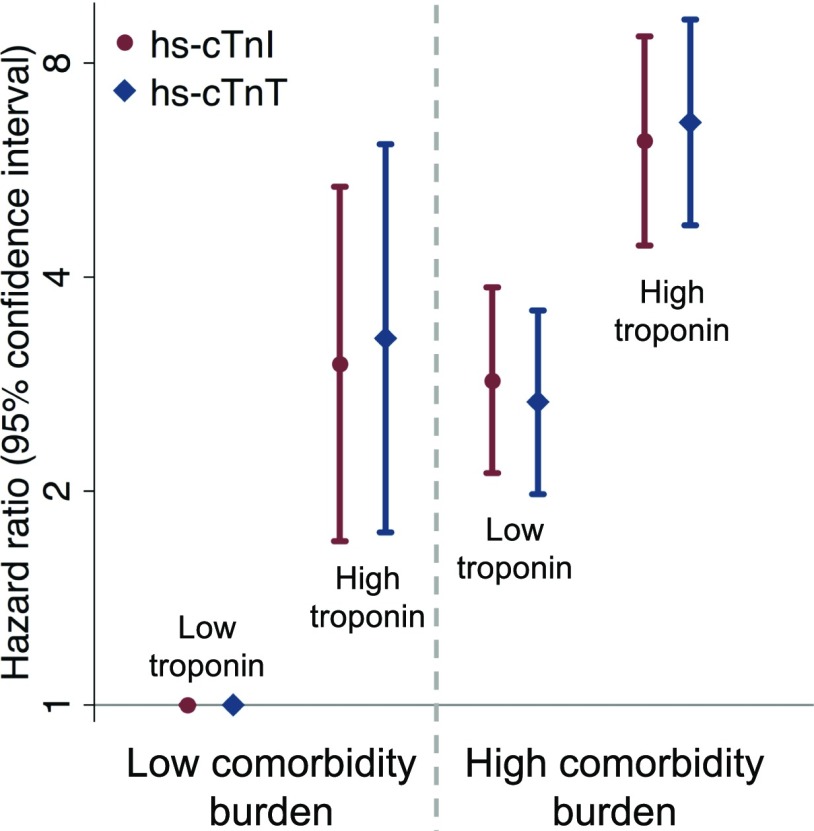
When considered jointly, both high hs-cTnI and high hs-cTnT added more prognostic information beyond comorbidity burden (Fig. 3 and Table 3). Models including either hs-cTnI or hs-cTnT did not differ in their C-statistics compared with one another (all P > 0.05). The addition of either hs-cTnI or hs-cTnT to survival models with traditional risk factors and comorbidity burden predominantly reclassified down those who did not die during follow-up (Supplementary Table 5). Those with elevations in either troponin and a high comorbidity burden were at the highest mortality risk, while isolated high cardiac troponin or isolated comorbidity burden with low troponin identified an intermediate-risk group at comparable risk to one another (Fig. 3). No significant interactions were observed with male sex (all Pinteraction > 0.05).
Figure 3.
Hazard ratios (95% CIs) of cross categories of high hs-cTnI or hs-cTnT and high comorbidity burden with subsequent all-cause mortality risk adjusted for traditional cardiovascular risk factors (age, male sex, race-center, smoking, systolic blood pressure, diastolic blood pressure, antihypertensive medication use, total cholesterol, HDL cholesterol, cholesterol-lowering medication use, and hemoglobin A1c).
Table 3.
C-statistics of models of all-cause mortality sequentially adding hs-cTnI or hs-cTnT to a base model including comorbidity burden
| C-statistic (95% CI) | ΔC-statistic (95% CI) | |
|---|---|---|
| Base model* with comorbidity burden (≥3 comorbidities) | 0.7024 (0.6781, 0.7267) | — |
| hs-cTnI ≥85th percentile | 0.7170 (0.6925, 0.7415) | 0.0146 (0.0051, 0.0242)† |
| log2(hs-cTnI) | 0.7243 (0.6999, 0.7486) | 0.0219 (0.0113, 0.0325)† |
| hs-cTnT ≥85th percentile | 0.7220 (0.6977, 0.7464)a | 0.0197 (0.0091, 0.0302)† |
| log2(hs-cTnT) | 0.7344 (0.7103, 0.7584)b | 0.0320 (0.0180, 0.0459)† |
| Base model* with comorbidity burden (≥3 comorbidities or dementia or frailty) | 0.7046 (0.6804, 0.7287) | — |
| hs-cTnI ≥85th percentile | 0.7201 (0.6958, 0.7445) | 0.0156 (0.0060, 0.0252)† |
| log2(hs-cTnI) | 0.7269 (0.7027, 0.7511) | 0.0223 (0.0117, 0.0330)† |
| hs-cTnT ≥85th percentile | 0.7238 (0.6995, 0.7480)c | 0.0192 (0.0088, 0.0296)† |
| log2(hs-cTnT) | 0.7363 (0.7123, 0.7602)d | 0.0317 (0.0180, 0.0455)† |
Age, male sex, race-center, current smoking, systolic blood pressure, diastolic blood pressure, antihypertensive medication use, total cholesterol, HDL cholesterol, cholesterol-lowering medication use, and hemoglobin A1c.
P < 0.01.
P = 0.35 compared with base model with comorbidity burden (≥3 comorbidities) + hs-cTnI ≥85th percentile.
P = 0.11 compared with base model with comorbidity burden (≥3 comorbidities) + log2(hs-cTnI).
P = 0.47 compared with base model with comorbidity burden (≥3 comorbidities or dementia or frailty) + hs-cTnI ≥85th percentile.
P = 0.13 compared with base model with comorbidity burden (≥3 comorbidities or dementia or frailty) + log2(hs-cTnI).
We observed similar findings when we assessed the use of troponin to risk stratify beyond health status levels defined by a high comorbidity burden or dementia or frailty. Those with high hs-cTnI or hs-cTnI and the presence of a high comorbidity burden or frailty or dementia were at the highest risk of mortality. Even among those with a low comorbidity burden, no dementia, and no frailty, high hs-cTnI or hs-cTnT was independently associated with a higher mortality risk (Supplementary Fig. 3).
When considered independently, coronary heart disease, heart failure, history of cancer, chronic kidney disease, depression, emphysema or chronic obstructive pulmonary disease, history of falls, severe hypoglycemia, and both geriatric syndromes were independently associated with elevated mortality risk after adjustment for cardiovascular risk factors (Table 4, model 2). The strongest associations were observed with dementia (hazard ratio 2.81 [95% CI 2.08, 3.80]), heart failure (2.48 [2.03, 3.04]), hypoglycemia (2.39 [1.58, 3.61]), frailty (2.35 [1.83, 3.01]), and chronic kidney disease (2.28 [1.84, 2.03]). These associations were attenuated but persisted after additional adjustment for hs-cTnI (Table 4, model 3a), hs-cTnT (Table 4, model 3b), or both troponins (Table 4, model 3c). Stroke, arthritis, hypertension, and incontinence were not independently associated with elevated mortality, even with minimal demographic adjustment (Table 4, model 1).
Table 4.
Hazard ratios (95% CIs) of the association of each comorbidity with all-cause mortality risk
| Model 1 | Model 2 | Model 3a | Model 3b | Model 3c | |
|---|---|---|---|---|---|
| ADA comorbidities | |||||
| Coronary heart disease | 1.92 (1.56, 2.38) | 1.87 (1.50, 2.33) | 1.55 (1.24, 1.94) | 1.60 (1.28, 2.00) | 1.50 (1.20, 1.87) |
| Stroke | 1.09 (0.73, 1.61) | 1.07 (0.72, 1.58) | 0.99 (0.67, 1.47) | 0.97 (0.65, 1.44) | 0.96 (0.65, 1.43) |
| Heart failure | 2.54 (2.08, 3.10) | 2.48 (2.03, 3.04) | 2.08 (1.69, 2.57) | 2.03 (1.65, 2.50) | 1.94 (1.57, 2.39) |
| Arthritis | 1.11 (0.88, 1.40) | 1.14 (0.90, 1.44) | 1.09 (0.87, 1.38) | 1.03 (0.81, 1.30) | 1.01 (0.80, 1.28) |
| Cancer | 1.65 (1.35, 2.02) | 1.65 (1.35, 2.03) | 1.66 (1.35, 2.03) | 1.52 (1.24, 1.87) | 1.55 (1.26, 1.90) |
| Chronic kidney disease | 2.32 (1.89, 2.86) | 2.28 (1.84, 2.82) | 1.83 (1.47, 2.28) | 1.67 (1.34, 2.09) | 1.58 (1.26, 1.98) |
| Depression | 1.69 (1.10, 2.61) | 1.64 (1.06, 2.54) | 1.58 (1.02, 2.46) | 1.67 (1.08, 2.59) | 1.58 (1.02, 2.46) |
| Emphysema or COPD | 1.92 (1.44, 2.55) | 1.90 (1.43, 2.54) | 1.85 (1.38, 2.47) | 1.72 (1.29, 2.30) | 1.78 (1.33, 2.37) |
| Falls | 1.82 (1.22, 2.72) | 1.75 (1.17, 2.62) | 1.47 (0.98, 2.21) | 1.64 (1.09, 2.45) | 1.55 (1.03, 2.33) |
| Hypoglycemia | 2.64 (1.75, 3.97) | 2.39 (1.58, 3.61) | 2.08 (1.37, 3.15) | 2.11 (1.39, 3.20) | 1.97 (1.30, 2.98) |
| Hypertension | 1.05 (0.79, 1.38) | 1.02 (0.58, 1.80) | 1.08 (0.61, 1.90) | 1.03 (0.58, 1.81) | 1.08 (0.61, 1.90) |
| Incontinence | 1.22 (0.93, 1.61) | 1.21 (0.91, 1.59) | 1.16 (0.88, 1.53) | 1.09 (0.82, 1.44) | 1.08 (0.81, 1.42) |
| Geriatric syndromes | |||||
| Dementia | 2.82 (2.09, 3.80) | 2.81 (2.08, 3.80) | 2.47 (1.83, 3.34) | 2.71 (2.00, 3.66) | 2.61 (1.93, 3.53) |
| Frailty | 2.27 (1.77, 2.91) | 2.35 (1.83, 3.01) | 1.95 (1.52, 2.52) | 1.76 (1.36, 2.27) | 1.67 (1.29, 2.16) |
Model 1: adjusted for age, sex, and race-center. Model 2: model 1 + current smoking, systolic blood pressure, diastolic blood pressure, antihypertensive medication use, total cholesterol, HDL cholesterol, cholesterol-lowering medication use, and hemoglobin A1c. Model 3a: model 2 + log2-transformed hs-cTnI with five linear equipercentile spline knots. Model 3b: model 2 + log2-transformed hs-cTnT with five linear equipercentile spline knots. Model 3c: model 2 + log2-transformed hs-cTnI with five linear equipercentile spline knots and log2-transformed hs-cTnT with five linear equipercentile spline knots. ADA, American Diabetes Association; COPD, chronic obstructive pulmonary disease.
Conclusions
Both high hs-cTnI and hs-cTnT are robustly associated with cardiovascular, respiratory, and microvascular comorbidities listed in the American Diabetes Association clinical guidelines. Levels of hs-cTnI and hs-cTnT are also predictive of mortality risk beyond traditional cardiovascular risk factors and comorbidity burden in this community-based population of older adults with diabetes.
Cardiovascular disease remains the leading cause of mortality in the U.S., despite advances in risk factor control (40,41). As the population ages, cardiovascular risk reduction in older adults continues to be of utmost importance. However, the heterogeneity in the health of older adults presents challenges to evaluating risk in older age. Clinical guidelines have advocated for a holistic approach to evaluation of older adults with diabetes, with consideration of functional and cognitive status and comorbidity burden in addition to traditional risk factors. For older adults, the potential side effects of glucose-lowering pharmacologic interventions may at times outweigh the benefits of intensive risk factor reduction, which typically manifests over a longer time scale (2,42).
Consistent with prior studies in other populations, we observed an increased risk of mortality among those with a higher comorbidity burden (43,44). The American Diabetes Association recommends more relaxed glycemic targets in older adults with high comorbidity burden. However, we observed only a modest difference in hemoglobin A1c among those with high comorbidity burden compared with those without. There could be a number of potential underlying reasons for this. First, it may not be sufficient to consider the number of comorbidities. This approach implicitly suggests an exchangeability between the distinct comorbidities. Physicians may be acting on a more holistic clinical impression of their patient, which may also include the presence of other comorbidities not explicitly named in the guidelines and a consideration of the relative severity of the comorbidities that are included. For example, two patients with three comorbidities may have very different risk/benefit profiles if one has heart disease, diabetes, and congestive heart failure and the other has osteoarthritis, controlled hypertension, and mild stress urinary incontinence. Second, this could reflect the challenges of operationalizing comorbidity burden to direct clinical care. Third, the achieved hemoglobin A1c may differ from the clinically intended target.
Our findings suggest that high-sensitivity cardiac troponins could be used to improve risk characterization and inform clinical management strategies in older adults with diabetes. In the general ambulatory population, hs-cTnI and hs-cTnT are detectable in 50–100% (45) and reflect cardiac-specific damage but are not disease specific. The use of objective biomarkers may address the lack of a uniform objective approach to assessing and incorporating comorbidity burden in treatment guidance. We observed that participants with a high hs-cTnI or hs-cTnT had high mortality risk compared with those without across levels of comorbidity burden. In addition to the cardiovascular comorbidities, elevations in troponin were commonly observed among participants with cognitive (dementia, stroke) or physical (frailty) dysfunction, which are key considerations in older age. Prior work in the ARIC Study has demonstrated that lifestyle factors, such as smoking, diet, and physical activity, are associated with changes in hs-cTnT (46), suggesting that lifestyle modifications may track with changes in troponin and be beneficial in reducing mortality risk.
Our results suggest that hs-cTnI or hs-cTnT could be used to refine comorbidity burden to identify subgroups of older adults at high mortality risk to improve the individualization of cardiovascular risk reduction strategies in older age. While hs-cTnI and hs-cTnT are more objective and concrete measures compared with comorbidity burden, they may not be able to completely replace the assessment of comorbidity status because those with high comorbidity burden and low hs-cTnI or hs-cTnT were also at an intermediate mortality risk. Furthermore, when considering individual comorbidities, additional adjustment for either or both of the cardiac troponins attenuated but did not completely explain the independent association of several comorbidities with mortality risk. Measurements of hs-cTnI or hs-cTnT are not subjected to concerns regarding patient recall of medical history, which has been shown to be poor in older adults (47). Our results suggest a role for measuring hs-cTnI or hs-cTnT to supplement the overall clinical impression of the patient.
The improvement in risk stratification using hs-cTnI or hs-cTnT appeared to be concentrated in downstratifying the risk among those who did not die as opposed to upstratifying the predicted risk for mortality among those who did die. This suggests that among older adults with diabetes, low hs-cTnI or hs-cTnT identifies the group that is at lower mortality risk, potentially reflecting better overall cardiovascular health. In this population, despite its age and multimorbidity, hs-cTnI or hs-cTnT might identify older patients with longer life expectancy and greater potential to benefit from more aggressive treatment of cardiovascular risk factors, such as more stringent glycemic control.
Few prior studies have focused on the mortality implications of novel cardiac biomarkers in the setting of diabetes, especially in older age. Our findings suggest that inclusion of troponins improved discrimination similarly to markers such as coronary artery calcium. In the Diabetes Heart Study (ages 34–86 years), inclusion of coronary calcium into logistic models for mortality (7.4 years of follow-up) significantly improved model discrimination (area under the curve 0.72 [95% CI 0.70, 0.74]) (48), similar to the improvements we observed among older adults in ARIC with time-to-death analyses. Inclusion of hs-cTnI and hs-cTnT may improve discrimination more than other biomarkers, such as CRP. A pooled analysis of participants with diabetes from four cohorts in the U.K. did not observe an improvement in C-statistics with the inclusion of CRP in cardiovascular mortality models (49).
There are some limitations to our study. First, some of the comorbidities were based on self-report, which may miss milder cases and be subjected to recall by the participant. This, however, could reflect usual clinical care, where patients often inform their physicians of their medical history. Conditions that were examined used validated, objective measures from laboratory results, medical records, and current medications. Second, we only followed participants for a median of 6.2 years. Time to benefit is an important clinical consideration in the management of older patients. Because many older adults are expected to live longer, better characterization of life expectancy is important in weighing the potential benefits of treatment against the possible risks.
There are a number of strengths in our study. To our knowledge, this is one of the few studies on comorbidities in older adults with rigorous measurements of key cardiovascular risk factors and biomarkers with follow-up for mortality. As one of few studies with simultaneous measurements of hs-cTnI and hs-cTnT, we were able to assess and compare the associations of both troponins in risk stratification. Moreover, the ARIC Study is a community-based cohort, with both black and white participants who underwent a comprehensive visit that included a physical examination, depression screening, and renal function tests, allowing us to objectively ascertain certain comorbidities. Finally, we were able to consider the contribution of dementia and frailty to mortality risk.
Cardiovascular, respiratory, and renal comorbidities were reflected by both hs-cTnI and hs-cTnT. Elevations in either of the cardiac troponins were associated with higher mortality risk across comorbidity levels, with or without accounting for dementia and frailty. These findings support the potential use of high-sensitivity troponins as objective measures of mortality risk to guide clinical care of older adults with diabetes.
Supplementary Material
Article Information
Acknowledgments. The authors thank the staff and participants of the ARIC Study for indispensable contributions.
Funding. The ARIC Study has been funded in whole or in part with federal funds from the National Heart, Lung, and Blood Institute (NHLBI), National Institutes of Health (NIH), Department of Health and Human Services, under contract numbers HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700004I, and HHSN268201700005I. Studies on cancer in ARIC are also supported by the National Cancer Institute (U01-CA-164975). Cancer incidence data have been provided by the Maryland Cancer Registry, Center for Cancer Surveillance and Control, Department of Mental Health and Hygiene. The authors acknowledge the State of Maryland, the Maryland Cigarette Restitution Fund, and the National Program of Cancer Registries of the Centers for Disease Control and Prevention for the funds that helped to support the availability of the cancer registry data. Neurocognitive data are collected by NIH (NHLBI, National Institute of Neurological Disorders and Stroke, National Institute on Aging, and National Institute on Deafness and Other Communication Disorders) grants U01-2U01-HL-096812, 2U01-HL-096814, 2U01-HL-096899, 2U01-HL-096902, and 2U01-HL-096917 and with previous brain MRI examinations funded by NHLBI grant R01-HL-70825. O.T. is supported by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grant F30-DK-120160. C.B. is supported by NHLBI grant R01-HL-134320. E.S. is supported by NIDDK grants K24-DK-106414 and R01-DK-089174. Reagents for the hs-cTnT assay were donated by Roche Diagnostics, and reagents for the hs-cTnI assay were donated by Abbott Diagnostics.
The content of this work is solely the responsibility of the authors and does not necessarily represent the official views of NIH.
Duality of Interest. K.M. has received nonfinancial support from Roche Diagnostics outside this work. R.H. has received grant support and consulting fees from Denka Seiken outside the submitted work. R.H. and C.B. are coinventors on a provisional patent (#61721475) titled Biomarkers To Improve Prediction of Heart Failure Risk filed by Roche Diagnostics and Baylor College of Medicine on their behalf. C.B. receives grant/research support from Abbott Diagnostics, Roche Diagnostics, and NIH and is a consultant for Abbott Diagnostics and Roche Diagnostics. E.S. has served on the advisory board for Roche Diagnostics. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. O.T. and N.D. conducted the analyses. O.T., N.D., K.M., J.C., A.R.S., R.H., X.J., B.G.W., C.B., and E.S. provided data interpretation and meaningful contributions to the revision of the manuscript. O.T. and E.S. designed the study and drafted the manuscript. O.T. and E.S. are the guarantors of this work and, as such, had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 3rd Annual Heart in Diabetes Conference, Philadelphia, PA, 12–14 July 2019.
Footnotes
This article contains Supplementary Data online at https://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc19-2043/-/DC1.
See accompanying article, p. 1172.
References
- 1.Moreno G, Mangione CM, Kimbro L, Vaisberg E; American Geriatrics Society Expert Panel on Care of Older Adults with Diabetes Mellitus . Guidelines abstracted from the American Geriatrics Society guidelines for improving the care of older adults with diabetes mellitus: 2013 update. J Am Geriatr Soc 2013;61:2020–2026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Diabetes Association Standards of Medical Care in Diabetes—2019. Diabetes Care 2019;42(Suppl. 1):S1–S193 [Google Scholar]
- 3.Qaseem A, Wilt TJ, Kansagara D, Horwitch C, Barry MJ, Forciea MA; Clinical Guidelines Committee of the American College of Physicians . Hemoglobin A1c targets for glycemic control with pharmacologic therapy for nonpregnant adults with type 2 diabetes mellitus: a guidance statement update from the American College of Physicians. Ann Intern Med 2018;168:569–576 [DOI] [PubMed] [Google Scholar]
- 4.LeRoith D, Biessels GJ, Braithwaite SS, et al. . Treatment of diabetes in older adults: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2019;104:1520–1574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marengoni A, Angleman S, Melis R, et al. . Aging with multimorbidity: a systematic review of the literature. Ageing Res Rev 2011;10:430–439 [DOI] [PubMed] [Google Scholar]
- 6.Munshi M. Treatment of type 2 diabetes mellitus in the older patient. In UpToDate. Nathan D, Schmader KE, Mulder JE, Eds. Waltham, MA, UpToDate Inc., 2019 [Google Scholar]
- 7.Kirkman MS, Briscoe VJ, Clark N, et al.; Consensus Development Conference on Diabetes and Older Adults . Diabetes in older adults: a consensus report. J Am Geriatr Soc 2012;60:2342–2356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–383 [DOI] [PubMed] [Google Scholar]
- 9.Huang ES, Zhang Q, Gandra N, Chin MH, Meltzer DO. The effect of comorbid illness and functional status on the expected benefits of intensive glucose control in older patients with type 2 diabetes: a decision analysis. Ann Intern Med 2008;149:11–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Medicare & Medicaid Services Chronic Conditions among Medicare Beneficiaries: A Methodological Overview [Internet], 2019. Available from www.ccwdata.org. Accessed 16 June 2019
- 11.Rich MW. Multimorbidity in Older Adults with Cardiovascular Disease: Expert Analysis - American College of Cardiology [Internet], 2016. Available from https://www.acc.org/latest-in-cardiology/articles/2016/09/16/10/01/multimorbidity-in-older-adults-with-cardiovascular-disease. Accessed 16 June 2019
- 12.Gagne JJ, Glynn RJ, Avorn J, Levin R, Schneeweiss S. A combined comorbidity score predicted mortality in elderly patients better than existing scores. J Clin Epidemiol 2011;64:749–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karlamangla A, Tinetti M, Guralnik J, Studenski S, Wetle T, Reuben D. Comorbidity in older adults: nosology of impairment, diseases, and conditions. J Gerontol A Biol Sci Med Sci 2007;62:296–300 [DOI] [PubMed] [Google Scholar]
- 14.National Center for Health Statistics Table 20. Leading causes of death and numbers of deaths, by age: United States, 1980 and 2016 [Internet], 2017. Available from https://www.cdc.gov/nchs/hus/contents2017.htm#020. Accessed 16 June 2019
- 15.Ritchie H, Rosner M. Causes of Death. Our World in Data [Internet], 2019. Available from https://ourworldindata.org/causes-of-death. Accessed 16 June 2019
- 16.Thygesen K, Alpert JS, Jaffe AS, et al.; Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction . Fourth universal definition of myocardial infarction (2018). J Am Coll Cardiol 2018;72:2231–226430153967 [Google Scholar]
- 17.Apple FS, Collinson PO; IFCC Task Force on Clinical Applications of Cardiac Biomarkers . Analytical characteristics of high-sensitivity cardiac troponin assays. Clin Chem 2012;58:54–61 [DOI] [PubMed] [Google Scholar]
- 18.Saunders JT, Nambi V, de Lemos JA, et al. . Cardiac troponin T measured by a highly sensitive assay predicts coronary heart disease, heart failure, and mortality in the Atherosclerosis Risk in Communities Study. Circulation 2011;123:1367–1376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.deFilippi CR, de Lemos JA, Christenson RH, et al. . Association of serial measures of cardiac troponin T using a sensitive assay with incident heart failure and cardiovascular mortality in older adults. JAMA 2010;304:2494–2502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Lemos JA, Drazner MH, Omland T, et al. . Association of troponin T detected with a highly sensitive assay and cardiac structure and mortality risk in the general population. JAMA 2010;304:2503–2512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Welsh P, Preiss D, Hayward C, et al. . Cardiac troponin T and troponin I in the general population. Circulation 2019;139:2754–2764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jia X, Sun W, Hoogeveen RC, et al. . High-sensitivity troponin I and incident coronary events, stroke, heart failure hospitalization, and mortality in the ARIC study. Circulation 2019;139:2642–2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Welsh P, Preiss D, Shah ASV, et al. . Comparison between high-sensitivity cardiac troponin T and cardiac troponin I in a large general population cohort. Clin Chem 2018;64:1607–1616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gore MO, Seliger SL, Defilippi CR, et al. . Age- and sex-dependent upper reference limits for the high-sensitivity cardiac troponin T assay. J Am Coll Cardiol 2014;63:1441–1448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hickman PE, Abhayaratna WP, Potter JM, Koerbin G. Age-related differences in hs-cTnI concentration in healthy adults. Clin Biochem 2019;69:26–29 [DOI] [PubMed] [Google Scholar]
- 26.Kelley WE, Januzzi JL, Christenson RH. Increases of cardiac troponin in conditions other than acute coronary syndrome and heart failure. Clin Chem 2009;55:2098–2112 [DOI] [PubMed] [Google Scholar]
- 27.ARIC Investigators The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol 1989;129:687–702 [PubMed] [Google Scholar]
- 28.Joshu CE, Barber JR, Coresh J, et al. . Enhancing the infrastructure of the Atherosclerosis Risk in Communities (ARIC) Study for cancer epidemiology research: ARIC cancer. Cancer Epidemiol Biomarkers Prev 2018;27:295–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lewinsohn PM, Seeley JR, Roberts RE, Allen NB. Center for Epidemiologic Studies Depression Scale (CES-D) as a screening instrument for depression among community-residing older adults. Psychol Aging 1997;12:277–287 [DOI] [PubMed] [Google Scholar]
- 30.Ginde AA, Blanc PG, Lieberman RM, Camargo CA Jr. Validation of ICD-9-CM coding algorithm for improved identification of hypoglycemia visits. BMC Endocr Disord 2008;8:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Juraschek SP, Daya N, Appel LJ, et al. . Orthostatic hypotension in middle-age and risk of falls. Am J Hypertens 2017;30:188–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee AK, Lee CJ, Huang ES, Sharrett AR, Coresh J, Selvin E. Risk factors for severe hypoglycemia in black and white adults with diabetes: the Atherosclerosis Risk in Communities (ARIC) Study. Diabetes Care 2017;40:1661–1667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Inker LA, Schmid CH, Tighiouart H, et al.; CKD-EPI Investigators . Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 2012;367:20–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Knopman DS, Gottesman RF, Sharrett AR, et al. . Mild cognitive impairment and dementia prevalence: the Atherosclerosis Risk in Communities Neurocognitive Study (ARIC-NCS). Alzheimers Dement (Amst) 2016;2:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fried LP, Tangen CM, Walston J, et al.; Cardiovascular Health Study Collaborative Research Group . Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56:M146–M156 [DOI] [PubMed] [Google Scholar]
- 36.Kucharska-Newton AM, Palta P, Burgard S, et al. . Operationalizing frailty in the Atherosclerosis Risk in Communities Study cohort. J Gerontol A Biol Sci Med Sci 2017;72:382–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parikh RH, Seliger SL, de Lemos J, et al. . Prognostic significance of high-sensitivity cardiac troponin T concentrations between the limit of blank and limit of detection in community-dwelling adults: a meta analysis. Clin Chem 2015;61:1524–1531 [DOI] [PubMed] [Google Scholar]
- 38.Chambless LE, Cummiskey CP, Cui G. Several methods to assess improvement in risk prediction models: extension to survival analysis. Stat Med 2011;30:22–38 [DOI] [PubMed] [Google Scholar]
- 39.Pencina MJ, D’Agostino RB Sr., Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med 2011;30:11–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Imperatore G, Cadwell BL, Geiss L, et al. . Thirty-year trends in cardiovascular risk factor levels among US adults with diabetes: National Health and Nutrition Examination Surveys, 1971-2000. Am J Epidemiol 2004;160:531–539 [DOI] [PubMed] [Google Scholar]
- 41.Wall HK, Ritchey MD, Gillespie C, Omura JD, Jamal A, George MG. Vital signs: prevalence of key cardiovascular disease risk factors for million hearts 2022 - United States, 2011-2016. MMWR Morb Mortal Wkly Rep 2018;67:983–991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Whelton PK, Carey RM, Aronow WS, et al. . 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines [published correction appears in Hypertension 2018;71:e140–e144]. Hypertension 2018;71:e13–e115 [DOI] [PubMed] [Google Scholar]
- 43.Deeg DJH, Portrait F, Lindeboom M. Health profiles and profile-specific health expectancies of older women and men: the Netherlands. J Women Aging 2002;14:27–46 [DOI] [PubMed] [Google Scholar]
- 44.Marengoni A, von Strauss E, Rizzuto D, Winblad B, Fratiglioni L. The impact of chronic multimorbidity and disability on functional decline and survival in elderly persons. A community-based, longitudinal study. J Intern Med 2009;265:288–295 [DOI] [PubMed] [Google Scholar]
- 45.Christenson RH, Jacobs E, Uettwiller-Geiger D, et al. . Comparison of 13 commercially available cardiac troponin assays in a multicenter north American study. J Appl Lab Med An AACC Publication 2017;1:544–561 [DOI] [PubMed] [Google Scholar]
- 46.Fretz A, McEvoy JW, Rebholz CM, et al. . Relation of lifestyle factors and life’s simple 7 score to temporal reduction in troponin levels measured by a high-sensitivity assay (from the Atherosclerosis Risk in Communities Study). Am J Cardiol 2018;121:430–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jones G, Tabassum V, Zarow GJ, Ala TA. The inability of older adults to recall their drugs and medical conditions. Drugs Aging 2015;32:329–336 [DOI] [PubMed] [Google Scholar]
- 48.Agarwal S, Morgan T, Herrington DM, et al. . Coronary calcium score and prediction of all-cause mortality in diabetes: the diabetes heart study. Diabetes Care 2011;34:1219–1224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kengne AP, Batty GD, Hamer M, Stamatakis E, Czernichow S. Association of C-reactive protein with cardiovascular disease mortality according to diabetes status: pooled analyses of 25,979 participants from four U.K. prospective cohort studies. Diabetes Care 2012;35:396–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.