Abstract
Background
Early diagnosis decreases the mortality of hepatocellular carcinoma (HCC). We aimed to investigate the usefulness of PIVKA‐II, AFP, AFP‐L3, CEA, and their combinations in the diagnosis of primary and metastatic HCC.
Methods
One hundred and twenty patients with primary HCC (PHC), 115 with metastatic HCC (MHC), 89 with chronic liver disease (CLD), and 116 healthy volunteers were included. The diagnostic values of each marker and their combinations for HCC diagnosis were represented by ROC curve analyses.
Results
PIVKA‐II, AFP, and AFP‐L3 levels in PHC group were higher than that in normal control, CLD, and MHC groups. CEA levels in MHC group were higher than that in the other three groups. When the four markers were analyzed individually, PIVKA‐II showed the highest positive rate in PHC group (76.7%) and CEA showed the highest positive rate in MHC group (69.6%). PIVKA‐ II showed the largest area under ROC curve (AUC = 0.835) to discriminate PHC group from CLD group. Combined PIVKA‐II with AFP‐L3 increased the AUC to 0.910. CEA showed the highest AUC (0.849) to discriminate MHC group from CLD group. Combined CEA with PIVKA‐II increased the AUC to 0.866. AFP‐L3 alone showed the highest AUC (0.890) to discriminate MHC group from PHC group. Combined PIVKA‐II with AFP‐L3, and CEA increased the AUC to 0.957.
Conclusion
PIVKA‐II, AFP‐L3, AFP, and CEA are effective biomarkers for the diagnosis of PHC and MHC. Their combinations could improve the diagnostic performance compared with each marker used alone in detecting PHC and MHC.
Keywords: AFP, AFP‐L3, CEA, hepatocellular carcinoma, PIVKA‐II
1. INTRODUCTION
Hepatocellular carcinoma (HCC) is one of the most common malignancies worldwide.1 Every year there are more than 500 000 newly diagnosed HCC cases.2 HCC has a variable geographical distribution, and the incidence in developing countries is two to three times higher than that in Western countries. In Eastern Asia and Middle Africa, the age‐adjusted incidence rate ranges from 20 to 28 cases per 105 people in men and around 55% of cases are in China.3 However, even in the western countries such as the United States (USA), the incidence of HCC is increasing,4 it is predicted to continue to increase in the following ten years.5
Hepatocellular carcinoma generally has no clinical symptoms in the early stage, and 2/3 of cases are found in the middle and advanced stage, when only about 20% of patients are suitable for surgical resection.6 Vascular invasion or extrahepatic tumor spread lead to no curative treatment options available. Metastatic HCC (MHC) is mainly determined by progression of the underlying liver disease rather than by the extrahepatic metastases.7 In China and Africa patients who present with symptoms usually die within 4 months8 but longer survival is possible in western countries.9 Therefore, screening and early diagnosis are vital to reduce the high mortality of HCC.
Several methods are available for HCC diagnosis. The recommended noninvasive methods include imaging techniques such as magnetic resonance imaging and the use of tumor markers such as AFP. However, in up to 40% of HCC patients, AFP levels are normal, especially in the early stage of the disease, which reflects low sensitivity. In order to improve the clinical outcome of patients, it is necessary to determine more reliable serum biomarkers.
PIVKA‐II is also known as des gamma carboxy prothrombin (DCP) and is an abnormal prothrombin molecule that is generated due to acquired defect in the posttranslational carboxylation of the prothrombin precursor in malignant cells. In 1984, Liebman et al10 used a radioimmunoassay to detect serum PIVKA‐II levels in patients with HCC and found that 91% of patients showed a significant increase. This finding has since been successfully applied in clinical practice.11 PIVKA‐II combined with Golgi protein 73 (GP73) showed higher accuracy than AFP in early HCC diagnosis.12 PIVKA‐II has been included in markers for auxiliary diagnosis of HCC in the Standardization of Diagnosis and Treatment for Hepatocellular Carcinoma (2011 Edition) issued by National Health Commission of the People's Republic of China. Prothrombin induced by vitamin K absence‐II (PIVKA‐II) and Lens culinaris‐agglutinin‐reactive fraction of AFP (AFP‐L3) have both been approved by FDA for risk stratification but not surveillance of hepatocellular carcinoma in the USA.13
Serum carcino‐embryonic antigen (CEA) is a relatively non‐specific antigen used in the clinical diagnosis of gastrointestinal cancer.14 The elevated level of CEA is found in some patients with poor HCC prognosis.15 Therefore, this marker may be useful for classification of prognosis. Many studies have reported the value of different tumor markers in the diagnosis of HCC, but there is currently no study using PIVKA‐II, AFP, AFP‐L3, CEA, and their combinations for the differential diagnosis of primary and metastatic HCC. Because of the high prevalence of HCC cases in China, seeking a set of diagnostic markers for HCC with better sensitivity and specificity than those in current use is particularly important. The aim of this study was to find an effective method to improve the early detection and accurate diagnosis of HCC, thus benefiting the prognosis of patients.
2. MATERIALS AND METHODS
2.1. Patients and grouping
Patients with HCC treated in Gansu Provincial Hospital from 2016 to 2018 were included. The inclusion criteria for patients with HCC were as follows: (a) 18‐85 years old; (b) Patients with pathologically confirmed HCC; (c) Patients meeting the Chinese guidelines Standardization of Diagnosis and Treatment for Hepatocellular Carcinoma (2017 Edition) as follows: (a) According to CT, MRI or ultrasound results, typical imaging lesions of HCC are seen, and typical blood flow changes occur in the lesions; (b) CT, MRI, or ultrasound suggest suspected small nodules, which are confirmed by PET examination; (d) Subjects had not received surgery, radiotherapy, chemotherapy, and other treatments. The exclusion criteria for patients and controls were as follows: (a) Subjects with missing laboratory detection data; (b) Subjects with missing clinical and medical history key data; (c) Subjects with severe hemolysis, microbial contamination, or jaundice; (d) Subjects that did not meet the requirements for sample collection or treatment; and (e) Subjects withdrawing from the trial based on the medical consideration by investigators. The included patients were grouped into PHC and MHC groups according to diagnosis. Patients with clinically confirmed MHC, mainly including: (a) Patients with a history of HCC and clinical manifestations of liver tumors; (b) Patients with imaging examination showing a solid liver space‐occupying lesion, most of which were scattered or multiple; and (c) Patients with liver metastasis found during surgery for primary disease, the diagnosis of which needed pathological examination. The patients with non‐viral liver diseases (including autoimmune liver disease, drug‐induced liver injury, and fatty liver) and hepatitis (mainly hepatitis B and hepatitis C) were included in the chronic disease group. The study was approved by the Research Ethics Committee of Gansu Provincial Hospital.
2.2. Clinical data collection and examination methods
Three microliters of venous blood was collected from the patients and healthy controls. The serum was separated and stored in −80°C freezer until use. PIVKA‐II, AFP, and CEA levels were measured in microparticle chemiluminescence instrument (Abbott I2000). AFP‐L3 was measured by enzyme‐linked immunosorbent assay (ELISA) (Shanghai Jonln Biotechnology). The cutoff values for the four markers were as follows: PIVKA‐II >39.54 mAu/mL, AFP >8.78 ng/mL, AFP‐L3 >7.26 ng/mL, and CEA >5.0 ng/mL.
2.3. Statistical analysis
Data were analyzed using SPSS 25.0 statistical software (IBM Corp.). Kruskal‐Wallis H Test was used for the comparison of the marker levels in four groups; the Bonferroni method was used for comparisons between each two groups, and P ≤ .05 was considered statistically significant. The diagnostic value of each index for PHC or MHC was represented by receiver operating characteristic (ROC) curve, and these were used to calculate the sensitivity, specificity, Youden index, and other indexes.
3. RESULTS
3.1. Demographic data of subjects
The baseline measurements for the four groups in this study are shown in Table 1. All of the measurements showed a significant difference between the four groups. Patients in PHC and MHC groups were older than those in CLD group and normal control group. It was reported that primary HCC can occur at any age, with the most patients between 40 and 59 years old and a male‐to‐female ratio of 2‐5:1. About 60% patients were male in chronic liver disease group while about 76% patients were male in PHC group. Thus, there was a slight deviation in patient age. The serum levels of various liver function indicators including ALT, AST, GGT, and ALB in patients with HCC were higher than those with chronic liver disease. The decreases in blood cell indexes including Plt, RBC, and Hb were also observed in patients with HCC.
Table 1.
Baseline data for the four groups included in the study
|
Normal control group (n = 116) |
CLD group (n = 89) |
PHC group (n = 120) |
MHC group (n = 115) |
P | |
|---|---|---|---|---|---|
| Age (years) | 43 (35‐56) | 47 (35‐57) | 56 (48‐66) | 63 (52‐70) | <.001 |
| Sex (n, %) | |||||
| Male | 50 (43.1) | 54 (60.6) | 92 (76.7) | 80 (69.6) | <.001 |
| Female | 66 (56.9) | 35 (39.4) | 28 (23.3) | 35 (30.4) | |
| ALT (U/L) | 18 (14‐25.75) | 32 (21‐48.5) | 52.4 (33.25‐101.1) | 37.4 (18.7‐85.2) | <.001 |
| AST (U/L) | 19 (16‐23) | 30 (21‐40) | 72.5 (37.4‐143.25) | 52.9 (31.05‐100.8) | <.001 |
| GGT (U/L) | 17 (14‐31) | 29.1 (17.95‐48.9) | 104.5 (39.86‐186.12) | 218 (45.12‐563.5) | <.001 |
| TBIL (μmol/L) | 14.75 (10.82‐19.45) | 21.2 (16.2‐29.9) | 31.7 (18.6‐57.55) | 20.88 (15.2‐35.3) | <.001 |
| DBIL (μmol/L) | 4.65 (3‐5.9) | 6.5 (5.15‐10.35) | 10.79 (7.01‐21.64) | 3.5 (2.7‐5.5) | <.001 |
| ALB (g/L) | 43.20 (41.5‐45.3) | 43.2 (36.65‐45.65) | 32.07 (28.53‐38.08) | 40.1 (36‐43.5) | <.001 |
| Plt (109/L) | 212 (176.25‐248.75) | 127 (70.5‐178) | 124.24 (72.64‐173.35) | 146 (93‐195) | <.001 |
| WBC (109/L) | 5.6 (4.9‐6.28) | 5 (3.96‐6.3) | 4 (2.8‐5.89) | 5.1 (3.9‐6.7) | <.001 |
| RBC (1012/L) | 4.74 (4.47‐5.01) | 4.57 (3.93‐5.03) | 3.13 (2.64‐3.93) | 3.81 (3.16‐4.53) | <.001 |
| Hb (g/L) | 146 (135.25‐154.75) | 144 (120‐161) | 89.15 (76‐123.88) | 104 (88‐131) | <.001 |
Abbreviations: ALB, albumin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; DBIL, direct bilirubin; GGT, gamma‐glutamyl transferase; Hb, hemoglobin; Plt, Platelets; RBC, red blood cells; TBIL, total direct and indirect bilirubin; WBC, white blood cells.
3.2. Comparison of the diagnostic values of four biomarkers in different groups
The levels of PIVKA‐II, AFP, and AFP‐L3 in PHC group were significantly higher than those in normal control, CLD, and MHC groups (P < .05; Table 2). The levels of CEA in MHC group were significantly higher than those in other three groups. The levels of PIVKA‐II in MHC group were significantly higher than those in normal control and CLD groups. AFP‐L3 and AFP levels in MHC group were significantly higher than those in normal control group (P < .05). When the four indexes were analyzed individually (Table 3), PIVKA‐II showed the highest positive rate in PHC group (76.7%) and CEA showed the highest positive rate in MHC group (69.6%). In the combined tests, PIVKA‐II or AFP‐L3, PIVKA‐II or AFP, and AFP or AFP‐L3 increased the positive rate of to 92.5%, 91.7%, and 91.7%, respectively, in PHC group.
Table 2.
The expression levels of four markers in different groups
| Groups | n | PIVKA‐II (mAU/mL) | AFP (ng/mL) | AFP‐L3 (ng/mL) | CEA (ng/mL) |
|---|---|---|---|---|---|
| Normal control | 116 | 21.9 (19.58‐24.97) | 2.71 (2.14‐3.77) | 3.44 (2.33‐5.09) | 1.56 (1.02‐1.90) |
| CLD | 89 | 23.4 (16.01‐39.17) | 4.7 (2.81‐7.29)a | 3.21 (2.0‐5.41) | 2.14 (1.3‐3.14)a |
| PHC | 120 | 2000 (43.44‐29771.36) a, b | 149.39 (8.01‐2000)a, b | 11.02 (6.83‐12.25)a, b | 2.90 (1.93‐4.29)a, b |
| MHC | 115 | 40.19 (27.56‐138.34)a, b, c | 4.27 (2.56‐10.57)a, c | 4.32 (3.22‐5.33)a, c | 10.04 (2.98‐782.40)a, b, c |
| H | 192.26 | 152.93 | 159.59 | 174.61 | |
| P | .000 | .000 | .000 | .000 |
vs normal control group.
vs chronic liver disease group.
vs PHC group, P ≤ .05.
Table 3.
Comparison of the positive rates of four markers in different groups
| Indexes | Normal control (n = 116) |
CLD (n = 89) |
PHC (n = 120) |
MHC (n = 115) |
χ 2 | P |
|---|---|---|---|---|---|---|
| PIVKA‐II | 2 (1.7) | 21 (23.6) | 92 (76.7) | 60 (52.5) | 154.856 | .000 |
| AFP | 0 (0.0) | 20 (22.5) | 89 (74.2) | 37 (32.2) | 177.3 | .000 |
| AFP‐L3 | 0 (0.0) | 18 (20.2) | 89 (74.2) | 15 (13.0) | 135.250 | .000 |
| CEA | 0 (0.0) | 10 (11.2) | 19 (15.8) | 80 (69.6) | 138.629 | .000 |
| PIVKA‐II or AFP | 2 (1.7) | 21 (46.1) | 110 (91.7) | 76 (66.1) | 115.921 | .000 |
| PIVKA‐II or AFP‐L3 | 2 (1.7) | 39 (43.8) | 111 (92.5) | 70 (60.9) | 113.386 | .000 |
| AFP or AFP‐L3 | 0 (0.0) | 38 (42.7) | 110 (91.7) | 49 (42.6) | 116.702 | .000 |
3.3. Evaluation of the diagnostic values of four biomarkers and their combinations in PHC group
The ROC curve analyses of the four markers in PHC group (compared with chronic liver disease group) were shown in Figure 1. When the four biomarkers were analyzed individually, PIVKA‐II, AFP, AFP‐L3, and CEA showed the area under ROC curve (AUC) of 0.835, 0.810, 0.807, and 0.625, respectively (Table 4). PIVKA‐II showed the highest AUC with a sensitivity of 0.835 and a specificity of 0.716. AFP‐L3 showed the highest sensitivity of 0.959 and AFP showed the highest specificity of 0.807. Combined PIVKA‐II with AFP‐L3 increased the AUC to 0.910 with increased sensitivity of 0.876 and specificity of 0.807. The combination of PIVKA‐II with AFP‐L3, and AFP could further increase AUC to 0.914 with an increased sensitivity of 1.000 but a decreased specificity of 0.625. The combination of four markers had no change in AUC (Table 4). These findings indicate that the combination of PIVKA‐II, AFP‐L3, and AFP could improve their abilities to discriminate patients with PHC and chronic liver diseases.
Figure 1.
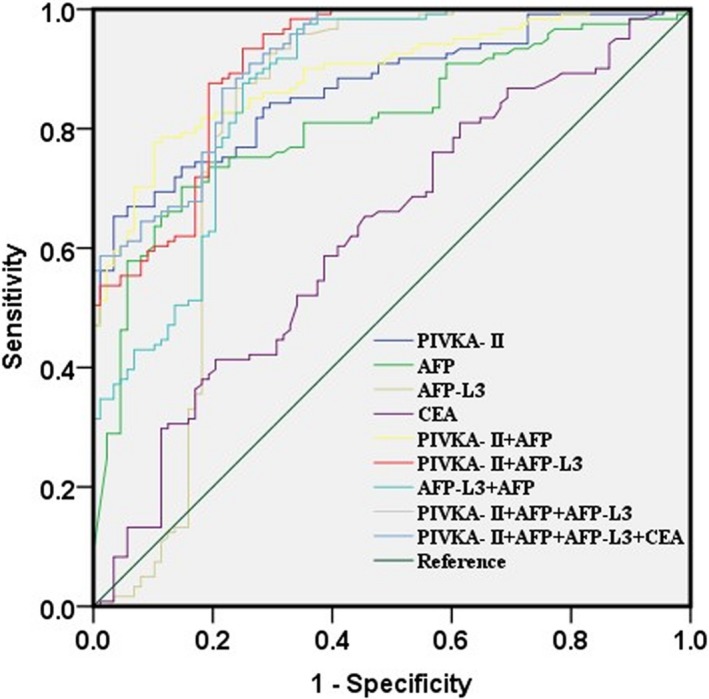
Receiver operating characteristic curves for PIVKA‐2, AFP, AFP‐L3, CEA, and their combinations in PHC group compared to CLD group
Table 4.
Receiver operating characteristic curve analyses of the diagnostic values of the four indexes alone and their combinations for distinguishing PHC group from CLD group
| Index | AUC | P | Cut‐off | Sensitivity | Specificity | Youden index |
|---|---|---|---|---|---|---|
| PIVKA‐II | 0.835 | .000 | 33.080 | 0.835 | 0.716 | 0.119 |
| AFP | 0.810 | .000 | 11.880 | 0.736 | 0.807 | 0.543 |
| AFP‐L3 | 0.807 | .000 | 4.367 | 0.959 | 0.670 | 0.629 |
| CEA | 0.625 | .000 | 2.520 | 0.587 | 0.614 | 0.201 |
| PIVKA‐II + AFP | 0.890 | .000 | 0.343 | 0.901 | 0.648 | 0.549 |
| PIVKA‐II + AFP‐L3 | 0.910 | .000 | 0.422 | 0.876 | 0.807 | 0.683 |
| AFP‐L3 + AFP | 0.865 | .000 | 0.291 | 0.975 | 0.648 | 0.623 |
| PIVKA‐II + AFP‐L3 + AFP | 0.914 | .000 | 0.217 | 1.000 | 0.625 | 0.625 |
| PIVKA‐II + AFP+AFP‐L3 + CEA | 0.914 | .000 | 0.420 | 0.868 | 0.784 | 0.652 |
3.4. Evaluation of the diagnostic values of four biomarkers and their combinations in MHC group
The ROC curve analyses of the four markers in PHC group (compared with chronic liver disease group) were shown in Figure 2. When the four biomarkers were analyzed individually, PIVKA‐II, AFP, AFP‐L3, and CEA showed the AUC of 0.744, 0.48, 0.588, and 0.849, respectively (Table 5). CEA showed the highest AUC with a sensitivity of 0.739 and a specificity of 0.888. PIVKA‐II showed the highest sensitivity of 0.896 and CEA showed the highest specificity of 0.888. Combined CEA with PIVKA‐II increased the AUC to 0.866 with increased specificity of 0.933 but decreased sensitivity of 0.696 (Table 5). These findings indicate that CEA, PIVKA‐II, and their combinations could improve their abilities to discriminate patients with MHC and chronic liver diseases.
Figure 2.
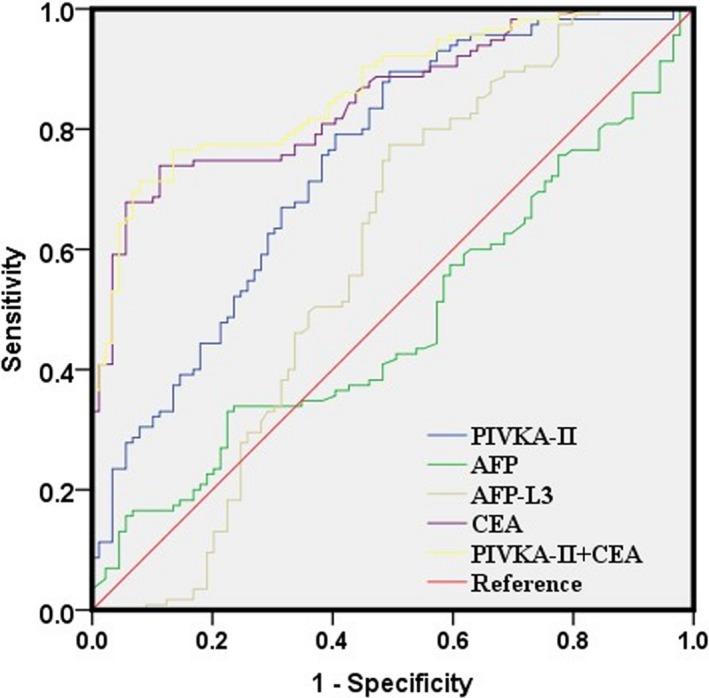
Receiver operating characteristic curves for PIVKA‐2, AFP, AFP‐L3, CEA, and their combinations in MHC group compared to CLD group
Table 5.
Receiver operating characteristic curve analyses of the diagnostic values of the four indexes alone and their combinations for distinguishing MHC group from CLD group
| Index | AUC | P | Cut‐off | Sensitivity | Specificity | Youden index |
|---|---|---|---|---|---|---|
| PIVKA‐II | 0.744 | .000 | 23.405 | 0.896 | 0.506 | 0.402 |
| AFP | 0.48 | .629 | 4.385 | 0.452 | 0.427 | 0.121 |
| AFP‐L3 | 0.588 | .032 | 3.205 | 0.774 | 0.494 | 0.268 |
| CEA | 0.849 | .000 | 3.995 | 0.739 | 0.888 | 0.627 |
| PIVKA‐II + CEA | 0.866 | .000 | 0.489 | 0.696 | 0.933 | 0.629 |
3.5. Evaluation of the diagnostic values of four biomarkers and their combinations in discriminating PHC and MHC groups
The ROC curve analyses of the four markers in MHC group (compared with PHC group) were shown in Figure 3. When the four biomarkers were analyzed individually, PIVKA‐II, AFP, AFP‐L3, and CEA showed the area under ROC curve (AUC) of 0.749, 0.784, 0.89, and 0.795, respectively (Table 6). AFP‐L3 showed the highest AUC with a sensitivity of 0.904 and a specificity of 0.725. AFP‐L3 showed the highest sensitivity of 0.904 and CEA showed the highest specificity of 0.892. Combined PIVKA‐II with AFP‐L3 increased the AUC to 0.917 with increased sensitivity of 0.922 and specificity of 0.792. Combined CEA with AFP‐L3 increased the AUC to 0.945 with increased sensitivity of 0.887 and specificity of 0.908. The combination of PIVKA‐II with AFP‐L3, and CEA could further increase AUC to 0.957 with a sensitivity of 0.861 and a specificity of 0.95 (Table 6). Adding AFP had no alteration in AUC although it increased the sensitivity but decreased the specificity. These findings indicate that the combination of PIVKA‐II, AFP‐L3, and CEA could improve their abilities to discriminate patients with MHC and PHC.
Figure 3.
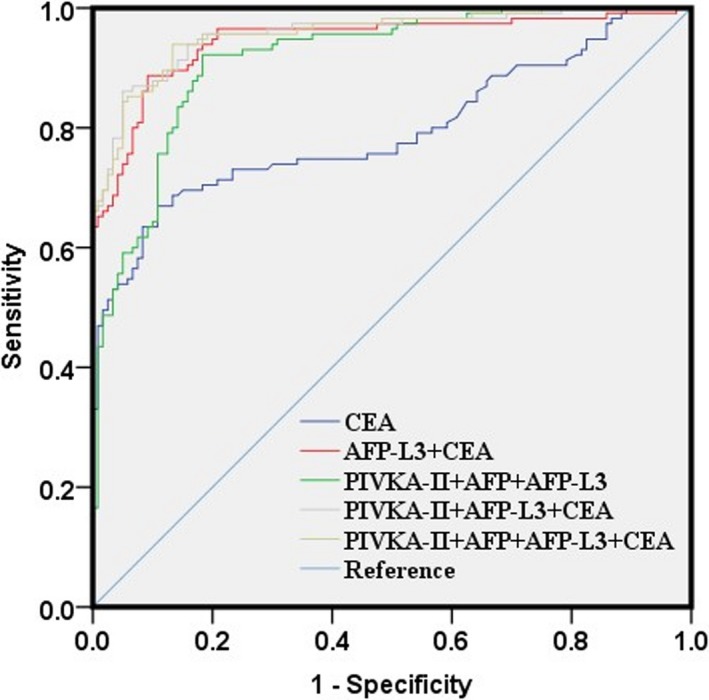
Receiver operating characteristic curves for PIVKA‐2, AFP, AFP‐L3, CEA, and their combinations in MHC group compared to PHC group
Table 6.
Receiver operating characteristic curve analyses of the diagnostic values of the four indexes alone and their combinations for distinguishing PHC group and MHC group
| Index | AUC | P | Cut‐off | Sensitivity | Specificity | Youden index |
|---|---|---|---|---|---|---|
| PIVKA‐ II | 0.749 | .000 | 296.110 | 0.887 | 0.600 | 0.487 |
| AFP | 0.784 | .000 | 18.105 | 0.826 | 0.708 | 0.534 |
| AFP‐L3 | 0.89 | .000 | 8.120 | 0.904 | 0.725 | 0.629 |
| CEA | 0.795 | .000 | 5.890 | 0.670 | 0.892 | 0.562 |
| PIVKA‐II + AFP | 0.805 | .000 | 0.629 | 0.800 | 0.725 | 0.525 |
| PIVKA‐II + AFP‐L3 | 0.917 | .000 | 0.344 | 0.922 | 0.792 | 0.714 |
| AFP‐L3 + AFP | 0.901 | .000 | 0.676 | 0.817 | 0.867 | 0.684 |
| PIVKA‐II + CEA | 0.870 | .000 | 0.531 | 0.661 | 0.942 | 0.603 |
| AFP‐L3 + CEA | 0.945 | .000 | 0.59 | 0.887 | 0.908 | 0.795 |
| AFP + CEA | 0.870 | .000 | 0.518 | 0.687 | 0.942 | 0.629 |
| PIVKA‐II + AFP‐L3 + CEA | 0.957 | .000 | 0.674 | 0.861 | 0.95 | 0.811 |
| PIVKA‐II + AFP‐L3 + AFP | 0.915 | .000 | 0.400 | 0.922 | 0.817 | 0.739 |
| PIVKA‐II + AFP‐L3 + AFP+CEA | 0.957 | .000 | 0.489 | 0.939 | 0.867 | 0.806 |
4. DISCUSSION
The early diagnosis of HCC is essential for curative interventions, which helps improve the prognosis and long‐term survival of patients. The previous studies have shown that PIVKA‐II is helpful for the diagnosis of HCC.1, 16 Moreover, several studies have shown that AFP‐L3 could improve the detection rate of HCC when it is combined with PIVKA‐II.17, 18 Lim et al18 also suggest that combining AFP, AFP‐L3, and PIVKA‐II improves the diagnostic accuracy for HCC among cirrhotic patients compared with using each marker individually. According to the Guidelines of Japan, AFP, AFP‐L3, and PIVKA‐II are recommended as serological biomarkers in clinical settings and these markers are routinely used to screen for HCC.19
In this study, we evaluated the effectiveness of four markers including PIVKA‐II, AFP‐L3, AFP, and CEA in discriminating patients with PHC, MHC, and chronic liver disease. These four markers were analyzed and compared either alone or in combination. As expected, the levels of all four markers were significantly elevated in patients with HCC compared to those with chronic liver disease. PIVKA‐II showed the highest positive rate in PHC group and CEA showed the highest positive rate in MHC group. When these markers were combined, the positive rate of PIVKA‐II/AFP‐L3 in PHC group was increased compared with PIVKA‐II or AFP‐L3 alone. On the contrary, while the positive rate of PIVKA‐II/AFP‐L3 or PIVKA‐II/AFP was lower than CEA alone in MHC group. These data suggest that PIVKA‐II, AFP‐L3, and AFP have complementary effects for the diagnosis of PHC. The reasonable use of these markers can improve the positive rate and accuracy of diagnosis. Moreover, it helps to reduce the rate of missed diagnosis and decrease the rate of misdiagnosis. This is consistent with the results reported in the literature.
PIVKA‐II has been found to be superior to AFP or AFP‐L3 in detecting PHC; this finding is consistent with those from several earlier studies.20, 21 We also compared the usefulness of AFP, AFP‐L3, and PIVKA‐II both individually and in combination in diagnosing PHC. We found that the combination of PIVKA‐II and AFP‐L3 was the most valuable panel for detecting PHC (AUC 0.910, sensitivity 0.876 specificity 0.800). This result is consistent with that reported in the literature 18, 22 showing that the combination of PIVKA‐II, AFP, or AFP‐L3 has a superior detection of PHC with no significant decrease in specificity in Asian population. Although the combination of three or four markers works better, the cost of examination also increases, which may reduce its potential of use.21 Other combinations of two or three markers did not provide superior diagnostic ability. Intriguingly, it has been reported that the changes of PIVKA‐II in PHC are not associated with AFP.23 Therefore, PIVKA‐II can improve the positive rate of diagnosis for AFP‐negative patients. The advantages of combined test have also been confirmed in clinical practice. For example, AFP, AFP‐L3, and PIVKA‐II are included as serum biomarkers in the clinical settings of Japan National Health Insurance.16 In the future, the sample size will be further expanded, and the association of PIVKA‐II with staging, curative effect and prognosis in PHC will be further studied.
For the diagnosis of metastatic HCC, CEA alone and PIVKA‐II alone showed a better AUC and the combination of CEA and PIVKA‐II showed the highest diagnostic accuracy. However, CEA had a better specificity than PIVA‐II while PIVA‐II had a better sensitivity than CEA in MHC. These findings indicate that PIVKA‐II and CEA has an ability to discriminate patients with MHC and chronic liver disease. CEA is a broad‐spectrum tumor marker that is combined with other biomarkers to diagnose primary HCC.24 However, we found that CEA was significantly elevated in MHC, which is helpful for differential diagnosis between primary and metastatic HCC. This is consistent with the findings from Huang et al25 showing that the patients with distant metastasis have high CEA levels than those without distant metastases. In addition, AFP‐L3 showed a sensitivity of 0.890 for distinguishing MHC and PHC groups, which was slightly higher than that previously reported in the Chinese population.26 The combination of AFP‐L3 and CEA was better to discriminate patients with MHC and PHC. (AUC 0.945, sensitivity 0.887, specificity 0.908). Overall, the sensitivity, specificity, and AUC for combined use were higher than those used alone for distinguishing MHC and PHC groups.
In conclusion, a combination of four biomarkers including AFP, PIVKA‐II, AFP‐L3 and CEA showed better accuracy than either marker alone in distinguishing patients with MHC, PHC, and chronic liver disease. The detection methods are simple, stable and reliable, and thus these markers are suitable for application in hospitals at all levels.
CONFLICTS OF INTEREST
The authors have no conflicts of interest to be declared.
ACKNOWLEDGMENTS
We thank the members of Department of Clinical Laboratory in Gansu Provincial Hospital for collecting clinical samples, providing technical assistance, and performing statistical analyses.
Qi F, Zhou A, Yan L, et al. The diagnostic value of PIVKA‐II, AFP, AFP‐L3, CEA, and their combinations in primary and metastatic hepatocellular carcinoma. J Clin Lab Anal. 2020;34:e23158 10.1002/jcla.23158
Qi and Zhou equally contributed to this work.
Funding information
The study was funded by Scientific Research Fund Project of Gansu Provincial Hospital (17GSSY1‐6), Key Laboratory of Molecular Diagnostics and Precision Medicine for Surgical Oncology in Gansu Province (2019GSZDSYS01).
Contributor Information
Lianhua Wei, Email: 107306723@qq.com.
Xu Zhang, Email: xuzhang@ujs.edu.cn.
REFERENCES
- 1. Mita Y, Aoyagi Y, Yanagi M, Suda T, Suzuki Y, Asakura H. The usefulness of determining des‐gamma‐carboxy prothrombin by sensitive enzyme immunoassay in the early diagnosis of patients with hepatocellular carcinoma. Cancer. 1998;82(9):1643‐1648. [DOI] [PubMed] [Google Scholar]
- 2. Teoh JYC, Hirai HW, Ho JMW, Chan FCH, Tsoi KKF, Ng CF. Global incidence of prostate cancer in developing and developed countries with changing age structures. PLoS One. 2019;14(10):e0221775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kudo M, Kitano M, Sakurai T, Nishida N. General rules for the clinical and pathological study of primary liver cancer, nationwide follow‐up survey and clinical practice guidelines: the outstanding achievements of the liver cancer study group of Japan. Dig Dis. 2015;33(6):765‐770. [DOI] [PubMed] [Google Scholar]
- 4. El‐Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;340(10):745‐750. [DOI] [PubMed] [Google Scholar]
- 5. Estes C, Anstee QM, Arias‐Loste MT, et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016–2030. J hepatology. 2018;69(4):896‐904. [DOI] [PubMed] [Google Scholar]
- 6. Liu C, Yang S, Wang K, et al. Alkaloids from traditional Chinese medicine against hepatocellular carcinoma. Biomed Pharmacother. 2019;120:109543. [DOI] [PubMed] [Google Scholar]
- 7. Bettinger D, Spode R, Glaser N, et al. Survival benefit of transarterial chemoembolization in patients with metastatic hepatocellular carcinoma: a single center experience. BMC Gastroenterol. 2017;17(1):98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kew MC, Geddes EW. Hepatocellular carcinoma in rural southern African blacks. Medicine (Baltimore). 1982;61(2):98‐108. [DOI] [PubMed] [Google Scholar]
- 9. Kudo M, Izumi N, Kubo S, et al. Report of the 20th nationwide follow‐up survey of primary liver cancer in Japan. Hepatol Res. 2019. 10.1111/hepr.13438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liebman HA, Furie BC, Tong MJ, et al. Des‐gamma‐carboxy (abnormal) prothrombin as a serum marker of primary hepatocellular carcinoma. N Engl J Med. 1984;310(22):1427‐1431. [DOI] [PubMed] [Google Scholar]
- 11. Gao J, Song P. Combination of triple biomarkers AFP, AFP‐L3, and PIVAKII for early detection of hepatocellular carcinoma in China: expectation. Drug Discov Ther. 2017;11(3):168‐169. [DOI] [PubMed] [Google Scholar]
- 12. Ismail MM, Morsi HK, Abdulateef NAB, Noaman MK, Abou El‐Ella GA. Evaluation of prothrombin induced by vitamin K absence, macrophage migration inhibitory factor and Golgi protein‐73 versus alpha fetoprotein for hepatocellular carcinoma diagnosis and surveillance. Scand J Clin Lab Invest. 2017;77(3):175‐183. [DOI] [PubMed] [Google Scholar]
- 13. Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American association for the study of liver diseases. Hepatology. 2018;68(2):723‐750. [DOI] [PubMed] [Google Scholar]
- 14. Yang W, Luo Y, Hu S, Li Y, Liu Q. Value of combined detection of serum carcino‐embryonic antigen, carbohydrate antigen 19–9 and cyclooxygenase‐2 in the diagnosis of colorectal cancer. Oncol Lett. 2018;16(2):1551‐1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yoshikawa M, Morine Y, Ikemoto T, et al. Elevated preoperative serum CEA level is associated with poor prognosis in patients with hepatocellular carcinoma through the epithelial‐mesenchymal transition. Anticancer Res. 2017;37(3):1169‐1175. [DOI] [PubMed] [Google Scholar]
- 16. Park SJ, Jang JY, Jeong SW, et al. Usefulness of AFP, AFP‐L3, and PIVKA‐II, and their combinations in diagnosing hepatocellular carcinoma. Medicine. 2017;96(11):e5811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hadziyannis E, Sialevris K, Georgiou A, Koskinas J. Analysis of serum α‐fetoprotein‐L3% and des‐γ carboxyprothrombin markers in cases with misleading hepatocellular carcinoma total α‐fetoprotein levels. Oncol Rep. 2013;29(2):835‐839. [DOI] [PubMed] [Google Scholar]
- 18. Choi JY. Diagnostic value of AFP‐L3 and PIVKA‐II in hepatocellular carcinoma according to total‐AFP. World J Gastroenterol. 2013;19(3):339‐346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lampignano R, Neumann MHD, Weber S, et al. Multicenter evaluation of circulating cell‐free DNA extraction and downstream analyses for the development of standardized (Pre)analytical Work flows. Clin Chem. 2019. 10.1373/clinchem.2019.306837 [DOI] [PubMed] [Google Scholar]
- 20. Lim TS, Kim DY, Han KH, et al. Combined use of AFP, PIVKA‐II, and AFP‐L3 as tumor markers enhances diagnostic accuracy for hepatocellular carcinoma in cirrhotic patients. Scand J Gastroenterol. 2016;51(3):344‐353. [DOI] [PubMed] [Google Scholar]
- 21. Volk ML, Hernandez JC, Su GL, Lok AS, Marrero JA. Risk factors for hepatocellular carcinoma may impair the performance of biomarkers: a comparison of AFP, DCP, and AFP‐L3. Cancer Biomark. 2007;3(2):79‐87. [DOI] [PubMed] [Google Scholar]
- 22. Koike Y, Shiratori Y, Sato S, et al. Des‐gamma‐carboxy prothrombin as a useful predisposing factor for the development of portal venous invasion in patients with hepatocellular carcinoma: a prospective analysis of 227 patients. Cancer. 2001;91(3):561‐569. [DOI] [PubMed] [Google Scholar]
- 23. Tian Z, Yu T, Wei H, Ning N. Clinical value of LHPP‐associated microRNAs combined with protein induced by vitamin K deficiency or antagonist‐II in the diagnosis of alpha‐fetoprotein‐negative hepatocellular carcinoma. J Clin Lab Anal. 2019:e23071 10.1002/jcla.23071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Edoo MIA, Chutturghoon VK, Wusu‐Ansah GK, et al. Serum biomarkers AFP, CEA and CA19‐9 combined detection for early diagnosis of hepatocellular carcinoma. Iran J Public Health. 2019;48(2):314‐322. [PMC free article] [PubMed] [Google Scholar]
- 25. Huang X, Li J, Wang F, Hao M. CT combined with tumor markers in the diagnosis and prognosis of hepatocellular carcinoma. J BUON. 2018;23(4):985‐991. [PubMed] [Google Scholar]
- 26. Wang Q, Chen Q, Zhang X, et al. Diagnostic value of gamma‐glutamyltransferase/aspartate aminotransferase ratio, protein induced by vitamin K absence or antagonist II, and alpha‐fetoprotein in hepatitis B virus‐related hepatocellular carcinoma. World J Gastroenterol. 2019;25(36):5515‐5529. [DOI] [PMC free article] [PubMed] [Google Scholar]


