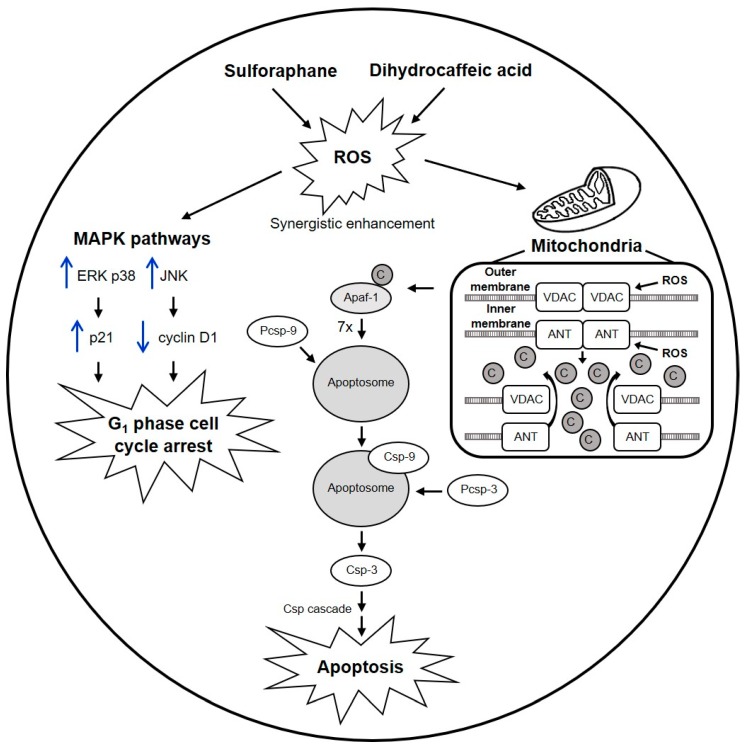
Figure 5.
Hypothetical model of the synergistic mechanism of sulforaphane and dihydrocaffeic acid against the viability of HT-29 colon cancer cells. Black arrows represent metabolic pathways, whereas blue arrows indicate up- or downregulation of proteins. Sulforaphane and dihydrocaffeic acid work together to enhance reactive oxygen species (ROS) production to levels that cannot be reached by either compound alone. ROS activate the mitogen-activated protein kinase (MAPK) pathways extracellular-regulated kinase (ERK), c-jun N-terminal kinase (JNK), and p38. ERK and p38 upregulate p21, while JNK downregulates cyclin D1. The result of these events is cell-cycle arrest at G1 phase. Simultaneously, ROS attacks mitochondria, specifically the voltage-dependent anion channel (VDAC) and adenine nucleotide translocator (ANT) proteins, which are located in the outer and inner membranes, respectively. This facilitates pore opening, causing the release of cytochrome c (C) into the cytoplasm. Then, cytochrome c initiates the apoptotic intrinsic pathway, where (1) cytochrome c binds with apoptosis protease activating factor-1 (Apaf-1) to form a binary complex; (2) seven Apaf-1/cytochrome c complexes assemble to form the apoptosome; and (3) the apoptosome recruits inactive procaspase-9 (Pcsp-9), facilitating its autocleavage into its active form caspase-9 (Csp-9). Csp-9, still bound to the apoptosome, cleaves procaspase-3 (Pcsp-3) into its active form caspase-3 (Csp-3). Finally, Csp-3 initiates a caspase cascade (Csp cascade) that leads to apoptosis.