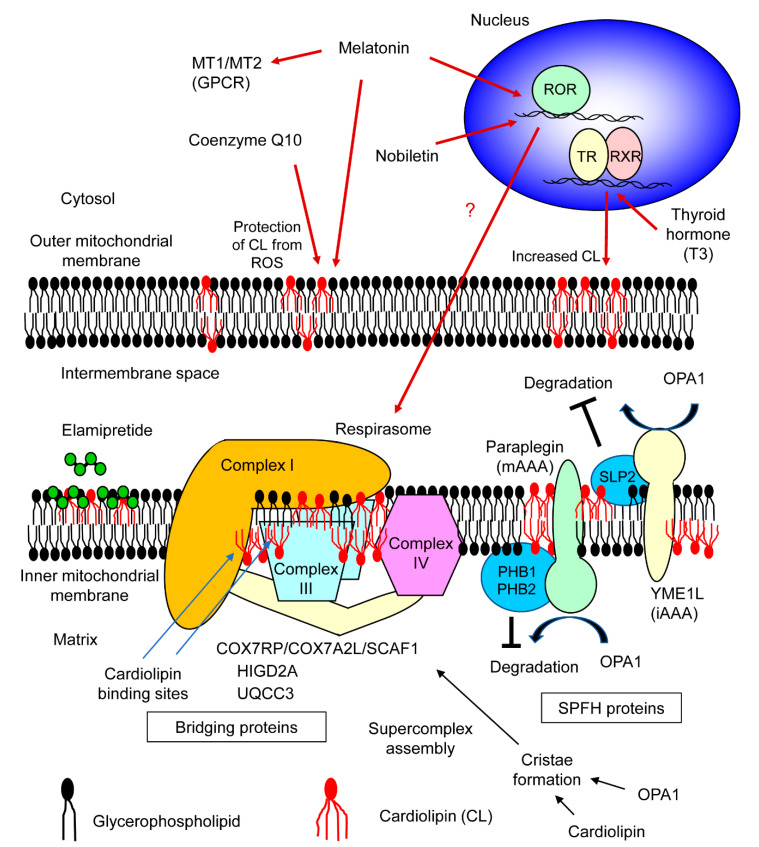
Figure 1.
Molecules involved in mitochondrial respiratory chain supercomplex assembly. The respiratory chain supercomplex assembly factors and molecules affecting supercomplex formation which are reported to function in mitochondria of vertebrates are shown. Cardiolipin (CL), a phospholipid with four acyl chains, directly binds respiratory chain complexes on the cardiolipin binding sites in each complex. Cardiolipin may indirectly support mitochondrial respiratory chain supercomplex formation by maintaining cristae structure. COX7RP (cytochrome c oxidase subunit 7a-related polypeptide; also known as COX7A2L/SCAF1), HIGD2A (HIG1 hypoxia inducible domain family, member 2A), and UQCC3 (ubiquinol-cytochrome c reductase complex assembly factor 3) function as bridging proteins by direct binding with each respiratory chain complex. PHB1 (prohibitin 1), PHB2 (prohibitin 2), and SLP2 (stomatin-like protein 2) are SPFH (stomatin, prohibitin, flotillin, and Hflk/C) proteins which inhibit activity of proteases, mAAA (a member of AAA (ATPase associated with diverse cellular activities) proteins with enzymatic sites in the matrix of mitochondria) and iAAA (a member of AAA proteins with enzymatic sites in the intermembrane space of mitochondria), causing degradation of OPA1 (optic atrophy 1) responsible for the maintenance of cristae structure. Five bioactive small molecules are also shown. Coenzyme Q10 and melatonin are antioxidants that protect cardiolipin from reactive oxygen species (ROS). Functions of melatonin are also mediated by its G-protein coupled receptors (MT1 and MT2) or by nuclear receptor ROR (retinoid acid receptor-related orphan receptor). Nobiletin also activates ROR. T3 type of thyroid hormone binds to thyroid hormone receptor (TR). TR forms heterodimer with retinoid X receptor (RXR). T3 increases the amount of cardiolipin. Elamipretide is a synthetic tetrapeptide which modulates electrostatics of the mitochondrial inner membrane.