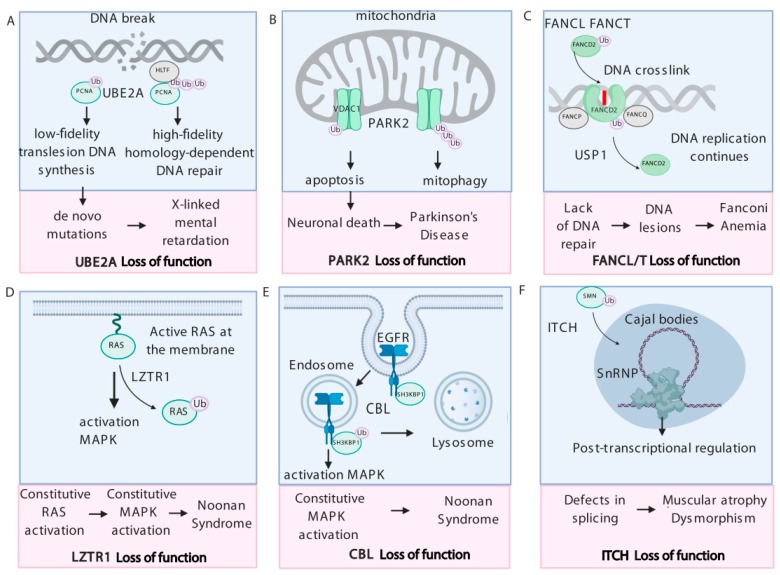
Figure 1.
The role of monoubiquitination in human diseases. (A) Ubiquitin-conjugating enzyme E2 A (UBE2A) loss of function impairs proliferating cell nuclear antigen (PCNA)-mediated DNA repair that partially explains developmental aspects of X-linked mental retardation. (B) Parkinson Protein 2 (PARK2) regulates mitophagy and apoptosis by controlling poly- and monoubiquitination of voltage-dependent anion-selective channel 1 (VDAC1). Dysregulation of VDAC1 ubiquitination contributes to the development of Parkinson’s disease. (C) Mutations in Fanconi Anemia complementation group L/T (FANCL/T) lead to DNA repair deficiency. Monoubiquitinated FA group D2 (FANCD2)/FA complementation group I (FANCI) heterodimer binds DNA, whereas deubiquitination of FANCD2 allows to re-start DNA replication. (D) The Rat sarcoma (RAS) GTPases are monoubiquitinated by the Leucine Zipper Like Transcription Regulator 1 (LZTR1)- Cullin 3 (CUL3) complex, inhibiting RAS association with the membrane and activating of RAS signaling. Hyperactivation of the mitogen activated protein kinase (MAPK) caused by LZTR1 loss of function leads to the Noonan syndrome phenotypes. (E) Casitas B-lineage lymphoma (CBL)-mediated monoubiquitination of SH3 domain-containing kinase-binding protein 1 (SH3KBP1) recruits active epidermal growth factor receptor (EGFR) for degradation. CBL mutations lead to up-regulation of the MAPK pathway that partially explains its contribution to the development of Noonan syndrome. (F) Mutations in E3 ubiquitin-protein ligase Itchy (ITCH) impair the monoubiquitination of survival motor neuron (SMN) that dysregulates translocation to Cajal bodies and affects post-transcriptional regulation of gene expression, linking ITCH loss of function to the development of spinal muscular atrophy.