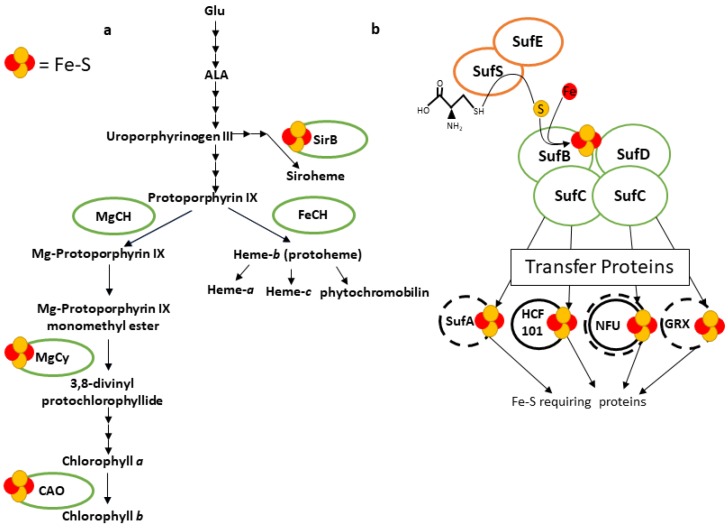
Figure 2.
Biosynthesis of Fe cofactors in the chloroplast. (a) Tetrapyrrole biosynthesis. Tetrapyrrole biosynthesis produces heme, siroheme, and chlorophyll. Enzymes requiring Fe–S clusters are denoted. Tetrapyrrole biosynthesis begins with Glutamate (Glu) which is used to form aminolevulinic acid (ALA) which is converted into protoporphyrin IX (PPIX). The pathway then splits to either produce heme by insertion of Fe by Ferrochelatase (FeCH) or chlorophyll a and b by insertion of Mg by Magnesium Chlelatase (MgCH). Chlorophyll biosynthesis is catalyzed, in part, by the enzymes, Mg Proto IX Monomethyl Ester Cyclase (MgCy) and Chlorophyllide A Oxygenase (CAO) which require Fe–S clusters. Siroheme cofactor production branches before protoporphyrin IX is produced and is catalyzed by Sirohydrochlorin Ferrochelatase (SirB). Each arrow signifies one enzymatic step. (b) Sulfur Utilization Factor (SUF) Fe–S assembly. Fe–S assembly begins with cysteine desulferase via SUFS and SUFE. The Fe–S cluster is produced on the SUFBCD scaffold and then may be transferred to candidate carrier molecules, including SUFA, High Chlorophyll Fluorescence 101 (HCF101), Nitrogen Fixation U-Like (NFU), and monothiol glutaredoxins (GRX), for delivery to target proteins. Enzymes necessary for cysteine desulfurase are orange, enzymes of the major scaffold are green, and transfer proteins are black. Dashed lines for the carrier proteins indicate biochemical evidence of their role. Solid lines indicate genetic evidence of their role.