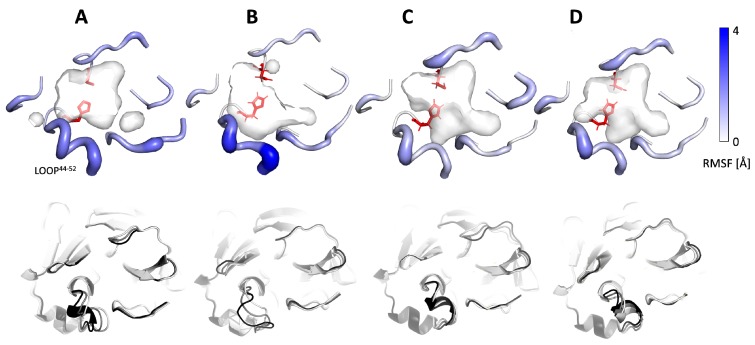
Figure 3.
Flexibility of loops surrounding the entrance to the binding cavity of (A) SARS-CoV-2 Mpro, (B) SARS-CoV Mpro, (C) SARS-CoV MproN3, and (D) SARS-CoV MproN3. For the picture clarity, only residues creating loops were shown. Upper row: RMSF data. The active site residues are shown as red sticks, and the A46S replacement between SARS-CoV and SARS-CoV-2 main proteases is shown as light blue sticks. The width and colour of the shown residues reflect the level of loop flexibility. The wider and darker residues are more flexible. Lower row: the results of normal mode analysis as a superposition of active site surroundings; structures are coloured white—initial conformation, black—final conformation, gray—transient conformation.