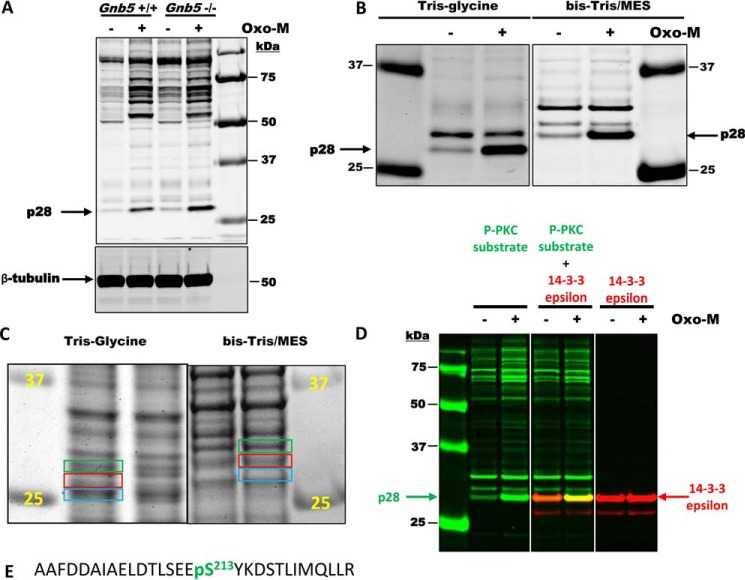
Figure 6.
PKC phosphorylates 14-3-3ϵ in MIN6 cells in a Gnb5-dependent manner. A, MIN6 cells were treated with or without 100 mm Oxo-M, and cell lysates were analyzed by Western blotting with anti-phospho-PKC substrate and β-tubulin antibodies. Note the strong PKC-mediated phosphorylation of the protein with an apparent molecular mass of 28 kDa (p28). This phosphorylation is increased ∼2-fold in Gnb5−/− cells. Sizes of protein standards are indicated on the right. B, MIN6 lysates were subjected to electrophoresis using two different buffer systems: Tris/glycine and BisTris/MES running buffer. Migration of p28 in these systems is different relative to protein markers. C, MIN6 lysates were subjected to electrophoresis using two different buffer systems, and the gels were stained with Coomassie Blue G250. Areas of the gels corresponding to p28 (red rectangles) were cut out and used for MS analysis. Areas right above and below (green and blue rectangles) were also analyzed by MS. D, confirmation of 14-3-3ϵ as a p28 PKC substrate. MIN6 lysates were subjected to electrophoresis using the BisTris/MES system. After transfer, the membrane was cut into three pieces and analyzed by Western blotting with anti-phospho-PKC substrate, 14-3-3ϵ, or a mixture of two antibodies. Rabbit anti-phospho-PKC substrate was visualized in the 680-nm channel (green). Mouse anti-14-3-3ϵ antibody was visualized in the 800-nm channel (red). E, the sequence of a 14-3-3ϵ phosphopeptide identified by MS analysis.