Abstract
Periodontitis is known to be initiated by periodontal microbiota derived from biofilm formation. The microbial dysbiotic changes in the biofilm trigger the host immune and inflammatory responses that can be both beneficial for the protection of the host from infection, and detrimental to the host, causing tissue destruction. During this process, recognition of Pathogen-Associated Molecular Patterns (PAMPs) by the host Pattern Recognition Receptors (PRRs) such as Toll-like receptors (TLRs) play an essential role in the host–microbe interaction and the subsequent innate as well as adaptive responses. If persistent, the adverse interaction triggered by the host immune response to the microorganisms associated with periodontal biofilms is a direct cause of periodontal inflammation and bone loss. A large number of T and B lymphocytes are infiltrated in the diseased gingival tissues, which can secrete inflammatory mediators and activate the osteolytic pathways, promoting periodontal inflammation and bone resorption. On the other hand, there is evidence showing that immune regulatory T and B cells are present in the diseased tissue and can be induced for the enhancement of their anti-inflammatory effects. Changes and distribution of the T/B lymphocytes phenotype seem to be a key determinant of the periodontal disease outcome, as the functional activities of these cells not only shape up the overall immune response pattern, but may directly regulate the osteoimmunological balance. Therefore, interventional strategies targeting TLR signaling and immune regulatory T/B cells may be a promising approach to rebalance the immune response and alleviate bone loss in periodontal disease. In this review, we will examine the etiological role of TLR signaling and immune cell osteoclastogenic activity in the pathogenesis of periodontitis. More importantly, the protective effects of immune regulatory lymphocytes, particularly the activation and functional role of IL-10 expressing regulatory B cells, will be discussed.
Keywords: periodontal disease, RANKL, Breg, Treg, TLR
1. Introduction
Periodontitis is one of the most predominant oral diseases that affect the majority of the population worldwide. Consistent with current knowledge of pathophysiology, in periodontal disease, three forms of periodontitis can be identified: necrotizing periodontitis [1], periodontitis as a manifestation of systemic disease [2], and what was previously considered separately to be “chronic” or “aggressive” form of the disease is now classified together as a new classification of “periodontitis” [3,4,5,6,7]. Periodontitis is known to be triggered by periodontal bacteria colonized at the host–microbe interface [8]. Various studies have identified keystone microbial species that are strongly associated with the onset and progress of periodontitis, such as Porphyromonas gingivalis (P. gingivalis) [9], Aggregatibacter actinomycetemcomitans (A. actinomycetemcomitans) [10], Tannerella forsythia (T. forsythia) [11], Fusobacterium nucleatum (F. nucleatum) [12], Treponema denticola (T. denticola) [13], and Actinomyces viscosus (A. viscosus) [14].
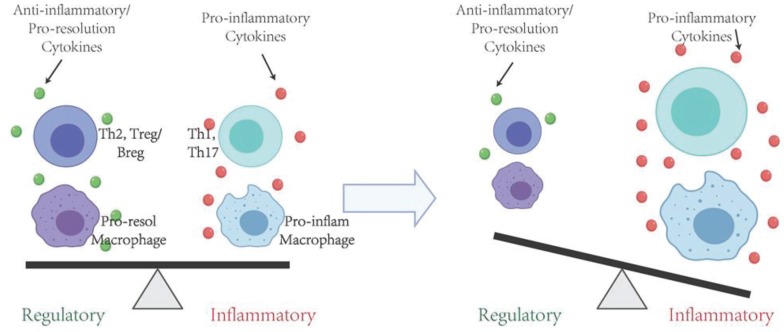
Although certain bacteria are considered "pathogens" due to their strong association with periodontal disease, they are also found in healthy sites of diseased patients or periodontal sites of healthy individuals. Therefore, none of these bacteria can be singled out as the cause of the periodontal disease because they have to adapt into the biofilm to form an organized microbial community, evolving towards a dysbiotic microbiota, eventually causing heightened periodontal inflammation and tissue destruction. While specific components or byproducts of bacteria, such as extracellular vesicles [15,16], enzymes (collagenase, protease and hyaluronidase) [17,18,19], toxins (such as leukotoxin) [20] and their metabolites (such as hydrogen sulfide) [21] may moderately disrupt periodontal tissue, the damage elicited by the adverse interaction between the subgingival biofilm and the host inflammatory immune response is considered the main cause of periodontal pathogenesis, with more substantial and persistent soft and hard tissue destruction [22,23]. There is now strong evidence that periodontitis is an inflammatory disease triggered by the host immune response to the microorganisms associated with periodontal biofilms, or their byproducts such as lipopolysaccharide (LPS), lipoprotein acids [24,25,26,27,28]. Such imbalance of pro-inflammatory and anti-inflammatory host cellular responses are considered a key element in disease pathogenesis and tissue damage (Figure 1).
Figure 1.
Immune responses directly contribute to the pathogenesis of periodontitis. A balanced pro- and anti-inflammatory responses need to be achieved to maintain tissue homeostasis. If the pro-inflammatory subtype of cells is predominantly persisted, it is inclined towards tissue destruction and bone resorption. Conversely, if the anti-inflammatory and pro-resolving lineages are predominantly developed in a timely fashion, inflammation will be controlled, and tissues will be repaired or regenerated.
There is a sequential event of the innate and adaptive immune responses leading to pathological alveolar bone resorption. After the acute inflammation is established, the recruitment of innate and adaptive immune cells and infiltration into the periodontal tissues mark a transition to the resolution phase or chronic inflammation. Affected by a series of environmental factors and the interactions of cellular and molecular components inherent to the host, different effector cell lineages may dominate the presence in the tissue, which determines the clinical outcome of the disease. If the pro-inflammatory subtype of cells is predominantly persisted, it is inclined towards tissue destruction and bone resorption. Conversely, if the anti-inflammatory and pro-regeneration lineages are predominantly developed in a timely fashion, inflammation will be resolved, and tissues will be repaired or regenerated.
2. Toll-Like Receptor (TLR) Signaling in the Etiology of Periodontitis
Ample studies have demonstrated that the initial host immune and inflammatory responses in periodontal disease were orchestrated by epithelial keratinocytes and fibroblasts of the periodontal connective tissue. Epithelial cells and gingival fibroblasts interact directly with microorganisms or their byproducts, generate and secrete molecular signals to trigger inflammation and attract immune cells [29,30]. Host cells recognize microorganisms through the interaction of Pattern Recognition Receptors (PRRs) that are constitutively expressed in the cell membrane of host cells, with the pathogen-associated molecular patterns (PAMP) presented by the microorganisms. These PAMPs are cell surface molecules that are preferentially related to pathogens and are not present in host cells, including lipopolysaccharide (LPS) (the main component of Gram-negative cell walls) and lipoteichoic acid (LTA) (the main component of cell walls of Gram-positive bacteria) [31]. The prototype membrane-bound PAMP receptor is in the Toll-like receptor (TLR) family, a type of PRRs that is considered to be the critical bridge linking innate and adaptive immunity [32]. Many studies have revealed that TLRs can recognize periodontal pathogens and regulate the host innate and adaptive immune response in periodontal disease [33,34,35]. To date, ten TLRs (TLR1 to TLR10) have been identified in humans. Each TLR is located in a specific compartment of the cell and can sense different PAMPs. T and B cells have been shown to express a number of TLRs (such as TLR 1, 2, 4, and 9) and respond to TLR ligands [36,37]. LPS is a potent ligand for TLR4 [38,39]. Lipoproteins produced by periodontal bacteria and P. gingivalis LPS can be recognized as ligands for TLR2 [40,41,42]. One study showed that P. gingivalis can activate both of the TLR2 and TLR4 pathways, leading to excessive production of pro-inflammatory cytokines and chemokines in monocytes [43]. TLR9 is known to be activated by microbial nucleic acids unmethylated CpG Oligodeoxynucleotide DNA (CpG) that can lead to periodontal inflammation and bone loss [44]. Stimulation by different types of bacteria in periodontal biofilm can induce the expression of different TLRs. The binding of pathogen-derived ligands to membrane-bound TLRs lead to the dimerization of TLRs and the subsequent activation of the downstream signaling pathways associated with inflammation and osteoclastogenesis.
The dimerization of TLR triggers the recruitment of various protein kinases in the cytoplasmic end of the receptor, and the classical inflammatory pathway is activated, leading to the upregulation of pro-inflammatory transcription factors (such as NFκB and AP-1) [45,46]. Various pro-inflammatory molecules, such as prostaglandin E-2 and leukotriene A-4 are produced. At the same time, TLR recognition of microbial ligands leads to the secretion of important molecular mediators of inflammation, including inflammatory cytokines (such as TNFα and IL-1β) and chemokines (such as CXCL8/IL-8, CCL2, CCL3 and CCL5), which promote the migration and infiltration of inflammatory cells into diseased periodontal tissues, such as dendritic cells, macrophages, and T and B cells [47,48]. Recently, a review of clinical human in vitro and in vivo studies reported a correlation between IFNγ in the crevicular fluid and periodontitis, potentially mediating the modulation of osteoclastogenesis [49]. During these processes, the infiltration of a large number of inflammatory cells is achieved by the degradation of the extravascular matrix around the blood vessels by enzymes such as matrix metalloproteinase (MMP) [50,51]. Under the influence of a large number of inflammatory signals, gingival fibroblasts and periodontal ligament fibroblasts destroy the fibrous components of the extracellular matrix of periodontal tissue by increasing the local production and activity of MMPs [52], leading to the destruction of periodontal soft tissues.
On the other hand, the role of TLR signaling on osteoclastogenesis and periodontal bone loss has also been extensively investigated. Ohgi et al. stimulated bone marrow macrophages (BMMs) with synthetic ligands for TLR2 (Pam3CSK4) or TLR4 (Lipid A), with or without receptor activator of nuclear factor kappa-B ligand (RANKL), and assessed for osteoclastogenesis by tartrate-resistant acid phosphatase (TRAP) staining, and found that osteoclastogenesis is promoted under the coexistence of oxidized low-density lipoprotein by TLR2-induced upregulation of Lectin-like oxidized low-density lipoprotein receptor 1 in mouse bone marrow cells [53]. Kishimoto et al. injected Escherichia coli peptidoglycan (PGN) or Staphylococcus aureus PGN with or without LPS into mouse gingiva, and histologically assessed alveolar bone resorption by TRAP staining. The results showed that Gram-positive or Gram-negative PGN worked synergistically with LPS to induce bone resorption and osteoclastogenesis, possibly by coordinating the effects of TLR2 and TLR4 signaling [54]. Zhang et al. investigated the direct effects of the periodontal pathogen P. gingivalis on osteoclast differentiation and showed that P. gingivalis differentially modulates RANKL-induced osteoclast formation through the modulation of TLR2/myeloid differentiation factor 88 (MyD88) [55]. Moreover, Yu et al. revealed significantly decreased bone loss and TRAP-positive cells in TLR2 KO mice as compared to WT mice in ligature-induced peri-implantitis and periodontitis, suggesting that TLR2 mediates bone loss in both peri-implantitis and periodontitis [56]. These findings strongly suggested the key roles of TLR signaling in the induction of periodontal inflammation and bone resorption.
3. Immune T and B Cells in Periodontitis Pathogenesis
Within the connective tissue of the periodontal infection site, there is a dense mononuclear inflammatory infiltrate containing all the cellular components of the immune network. In recent years, increasing evidence has substantiated that the inflammatory activation of the immune system in periodontitis cause heightened pathological osteoclastogenesis and alveolar bone destruction. Such close connection and interaction between the immune system and bone metabolism has been referred to as "osteoimmunology" [57,58,59]. Many studies have shown that the host immune response plays a key role in stimulating osteoclast differentiation and promoting bone resorption in periodontal disease [60,61,62,63,64].
It is now clear that the host response to bacteria involving activated T and B lymphocytes and these cells contribute to the pathogenesis of periodontitis bone resorption. Previous studies have shown that a large number of T and B lymphocytes were infiltrated in gingival tissue in an antigen-specific manner [65,66,67]. Specifically, key links between T cells and bone resorption have been established [68]. Yoshie and colleagues [69] implicated the pathogenesis of activated T lymphocytes and periodontal disease. Similar results have been observed in experimental mouse models, suggesting that T cells and their response to oral infections by P. gingivalis help advance bone remodeling in the direction of net bone loss [70]. Kawai and colleagues used an experimental adoptive T cell transfer periodontal disease model to show that Th1 cells combined with B7 co-stimulation appeared to trigger inflammatory bone resorption [71]. This bone resorption can be eliminated by the use of a fusion protein (CTLA4Ig), which interferes with the co-stimulatory interaction (CD28 and B7) between T lymphocytes and antigen-presenting cells, further substantiating the direct involvement of the immune response in the induction of periodontal bone resorption [72,73]. Conversely, Yamashita and colleagues transferred A. actinomycetemcomitans-specific Th2-cell clones to normal heterozygous rats followed by infection with A. actinomycetemcomitans, and found that bone loss was significantly reduced in the recipients of A. actinomycetemcomitans-specific Th2 cells when compared with the other infected group, which supports the notion that Th2 cells appear to interfere with periodontal bone loss [74,75]. Accumulated data also support B cell involvement in the induction of bone resorption [76]. Kozuka et al. discovered the histopathological changes in normal mice, in SCID mice that lack both B and T cells, and in B cell-reconstituted SCID mice, after repeated injections of LPS into the gingiva. As a result, the B cell-reconstituted SCID mice showed stronger inflammatory bone resorption than the SCID mice, which suggests that B cells promote inflammatory bone resorption [77]. Similarly, studies also demonstrated that adoptive transfer of antigen-specific B cells induce periodontal bone loss [78,79]. These in vivo adoptive transfer experiments of antigen-specific T cell clones [80] and antigen-specific B lymphocytes [78] firmly established the role of activated T and B cells in the periodontal bone resorption through activation and differentiation of osteoclast precursor cells along the alveolar bone surface of animals receiving antigen-specific lymphocytes.
4. Immune Cell RANKL Activity in the Progression and Pathogenesis of Periodontitis
Despite the complexity of the input signals, the skeletal system and bones have a relatively simple signal transduction system, capable of "sensing" a variety of stimuli, and the effector mechanism is controlled by a limited number of "key effector" pathways. In the context of alveolar bone, the system that controls bone metabolic balance includes the RANKL/RANK/OPG system secreted and recognized by specialized bone metabolism effector osteoblasts and osteoclasts [81,82].
Receptor activator of nuclear factor κB ligand (RANKL) is a cytokine that is a member of the TNF family. Its predominant function is to stimulate osteoclast differentiation, cell fusion and activation, leading to bone resorption through calcium-dependent activation of the NFATc1 gene transcription [83]. Therefore, RANKL is a major activator of osteoclasts and is a molecular signal that directly causes bone resorption. RANKL comes in two forms: (1) membrane-bound RANKL (mRANKL) and (2) soluble RANKL (sRANKL) [84]. Increasing evidence suggests that sRANKL is more potent in triggering osteoclast formation than mRANKL [85]. RANKL interacts with its receptor RANK on the surface of osteoclasts and osteoclast precursors, triggering their recruitment to the bone surface, cell fusion and activation. Many animal model experiments have shown that alveolar bone resorption can be prevented by selective inhibition of the RANKL/RANK axis [86,87].
Osteoprotectin (OPG) is a soluble protein that is upregulated under inflammatory conditions. As a decoy receptor, it has the function of blocking the biological activity of RANKL through competitive inhibition by limiting the ability of RANKL to bind to RANK. The quotient or ratio of RANKL and OPG determines whether conditions are suitable for bone deposition or bone resorption at any particular time. Higher RANKL/OPG ratios create conditions conducive to bone resorption, while lower RANKL/OPG ratios facilitate bone deposition [88,89]. During the bacterial-induced inflammation in experimental periodontitis, a net increase in the RANKL/OPG ratio has been shown to lead to the increased activation of osteoclasts and elevated occurrence of bone resorption [90]. There is similar evidence in the case of peri-implantitis [91], as well as pathological bone resorption of the periapical lesion due to dental pulp infection [92,93].
In periodontal disease, the inflammatory infiltration of T cells, B cells, macrophages, and neutrophils in gingival connective tissue is increased, accompanied with the increase of secretion of inflammatory mediators [94,95]. These inflammatory cells also interact with stromal cells, such as osteoblasts, periodontal ligament cells, and gingival fibroblasts. As RANKL-mediated osteoclast formation plays a key role in inflammatory bone resorption, its expression and production level were observed to increase significantly in periodontitis lesions [96]. Studies have shown that activated T and B lymphocytes are the major sources of RANKL in diseased periodontal tissues [97,98,99,100,101,102]. More than 90% of B cells recovered from human periodontal diseased tissue express RANKL, as well as about 54% of T cells [103]. These cells have also been tested in vitro to induce osteoclast differentiation and bone resorption [103,104,105]. In addition, both forms of sRANKL and mRANKL appear to be expressed from T and B cells [103]. Therefore, RANKL is now considered an important molecular signal that bridges immune response with bone metabolism.
Since the studies involving clinical tissue samples have shown that activated T and B cells produce increased RANKL in diseased sites, many researchers have sought to elucidate the mechanisms by which lymphocytes are activated and eventually contribute to RANKL-mediated bone resorption. Teng et al. used human CD4+ T cells isolated from local aggressive periodontitis patients and transferred them into NOD-SCID mice that have been orally colonized with A. actinomycetemcomitans, and found that the alveolar bone loss induced by transferred T cells was RANKL-dependent [106]. Other studies indicated that the inhibition RANKL activity by OPG reduced T cell-mediated experimental periodontal bone destruction in rodent models [103,107,108,109]. These findings further verified that antigen-specific T lymphocytes trigger bone destruction in periodontal tissues in a RANKL-dependent manner.
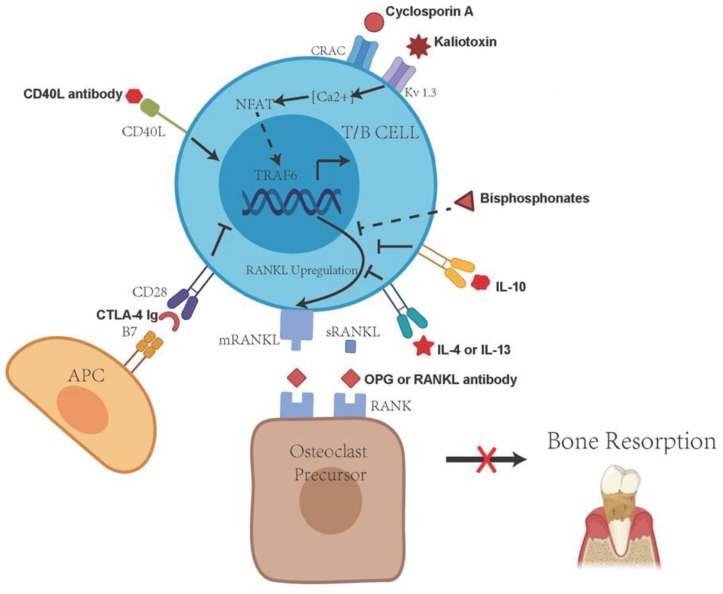
Antigen-specific T cells not only produce RANKL, but also induce B cell activation through CD40/CD40L interactions, leading to the production of RANKL by B cells [60]. In addition, there are studies showing that B cells stimulated by A. actinomycetemcomitans overexpress RANKL, and adoptive transfer of the antigen-specific B lymphocytes can promote periodontal bone resorption in mice injected with A. actinomycetemcomitans [78,79]. The observed periodontal bone resorption can be abolished by the administration of OPG-Fc in this B cell transfer model [78]. Studies of adoptive transfer of antigen-specific B cells into T cell deficient (congenital athymic) rats have shown that animals that receive antigen-specific RANKL-expressing B cells promoted the induction of osteoclasts in vitro and significantly increased experimentally induced bone resorption in vivo. Control animals receiving B cells with minimal RANKL expression did not show any bone resorption [105]. This indicates that antigen-specific RANKL-expressing B lymphocytes can promote periodontal bone resorption in the absence of T lymphocytes. Since the activated antigen-specific T and B cells are the main source of RANKL in periodontal disease, immunological interventions targeting the RANKL activities in these cells have been considered a viable approach to mitigate the immune-mediated RANKL-dependent periodontal bone loss (Figure 2).
Figure 2.
Potential immunological approaches to interfere with immune cell-mediated, RANKL-dependent periodontal bone resorption. The central target of these approaches is the inhibition of RANKL expression, secretion and interaction with RANK on osteoclast precursor cells. APC, antigen-presenting cell; CD40 L, CD40 ligand; CRAC, Ca2+ release-activated Ca2+ channels; IL-10, interleukin-10; mRANKL, membrane-bound RANKL; sRANKL, soluble RANKL; NFAT, nuclear factor of activated T cells; OPG, osteoprotegerin; RANK, receptor activator of nuclear factor κB; RANKL, receptor activator of nuclear factor κB ligand; TRAF6, tumor necrosis factor-receptor associated factor 6.
5. TLR Signaling and Regulation of RANKL Activity
Regulation of RANKL production and function by TLR signaling have long been demonstrated, suggesting that interactions between TLR and RANKL/RANKL system are associated with the mechanism underlying the pathogenesis of bacteria-mediated periodontal bone loss. Zhang et al. reported that TLR2-dependent modulation of osteoclastogenesis by P. gingivalis is achieved through differential induction of NFATc1 and NF-κB, and the subsequent RANKL modulation [55]. P. gingivalis LPS could also directly induce periosteal osteoclast formation and bone resorption by stimulating RANKL in osteoblasts via TLR2. This effect may not only play an important role in periodontal bone loss but also is indicated in the enhancement of bone resorptive condition seen in rheumatoid arthritis patients with concomitant periodontal disease [110].
Interestingly, studies have also shown that activation of TLR (especially TLR4 and TLR9) in early osteoclast precursors leads to inhibition of RANKL-induced osteoclast differentiation through IL-12 [111]. In human osteoclast precursor cell culture models, TLR ligands inhibit RANK expression by downregulating the cell surface expression of the M-CSF receptor c-Fms, thereby inhibiting osteoclast formation [112]. As components of Gram-negative bacteria, LPS and CpG-ODN trigger TLR4 and TLR9 at the site of infection, respectively, although the biological effects of these two components may change at different stages of infection [113]. The apparent differential effects of TLR signaling on immune cells vs. bone cells in RANKL-associated osteoclastogenesis may indicate unidentified feedback mechanisms in the homeostasis of bone metabolism and such details in the interaction of the osteo-immunological network are warranted for future investigations.
Depending on the model system, outcome measurement, and microenvironment involved in such studies, different findings have also been reported regarding the engagement of TLR2 and TLR4 in P. gingivalis vs. ligature-induced periodontal bone loss, indicating that P. gingivalis-induced periodontal bone resorption is TLR4-dependent, whereas ligation-induced periodontal bone resorption is neither TLR2- nor TLR4-dependent [114]. Furthermore, the effects of TLR signaling and on RANKL-dependent and -independent mechanisms have been proposed to induce osteoclastogenesis, and other endogenous factors such as miRNA, IL-22, M1/M2 macrophages, and memory B cells have been identified recently as potential contributors in the regulation of osteoclastogenesis and bone loss during periodontal disease pathogenesis [115].
6. Protective Effects of Immune Regulatory T Cells in the Inflammation and Periodontal Bone Resorption
Changes in T helper cell response seem to be a key determinant of periodontal bone loss, as this type of cell not only regulates the overall immune response pattern, but may directly interfere with the RANKL/OPG ratio [116]. Regulatory T cell (Treg) is a T helper lymphocyte associated with the secretion of anti-inflammatory cytokines and resolving molecular signals such as IL-10 and TGF-β. IL-10 secretion and CTLA4 co-receptor-mediated inhibition of lymphocyte activation are hallmark effector mechanisms of Treg function, leading to active and transient suppression of the immune response and restoration of tissue homeostasis. In vivo experimental data have correlated the presence of Tregs with reduced osteolytic progression in periodontal disease [117]. In an interesting in vivo experiment, it has been shown that endogenous Tregs recruited to the specific periodontal inflammation sites can effectively reduce production of inflammatory markers in periodontal tissues, limit bone resorption, and promote the establishment of a regenerative environment [118]. It is worth noting that the selective recruitment of Tregs into periodontal tissue is not associated with an increase in bacterial load or dampening the bacterial clearance mechanism. Such Tregs modulation hence provides an attractive possibility for safe local immune regulation, which may be explored as a potential future approach to enhance the efficacy of conventional periodontal therapy [118].
7. Protective Effects of Immune Regulatory B Cells in the Inflammation and Periodontal Bone Resorption
Generally, B cells are considered a source of terminal effector cells that produce antigen-specific antibodies against periodontal pathogens, and they participate in the host immune response to microorganisms that invade periodontal tissue [119]. However, this view is gradually changing as we gain more insights about the role of B cells in host innate and adaptive immunity. An asynchronous relationship identified between the clinical periodontitis index and the antibody concentration in the diseased sites suggests that B cell functions may be pleiotropic in regulating periodontal inflammation [120]. Accumulated reports now suggest that in addition to T helper cells, certain subsets of B cells may be another source of immune regulators of the host immune response [121]. As early as the 1990s, existence of B cells with immunosuppressive functions were suggested. Two animal studies showed that mice lacking B cells exacerbate the disease progression in experimental autoimmune encephalomyelitis (EAE) and chronic colitis, demonstrating the potential regulatory role of B cells in autoimmune inflammation [122,123]. In 2002, Mizoguchi et al. first used the term "regulatory B cells" to describe the B cells that suppress inflammatory bowel disease [124]. B-lineage cells with regulatory functions are now commonly referred to as "regulatory B cells", or Bregs [125,126,127,128]. Currently, Bregs have been shown to have varying degrees of immunosuppressive effects in autoimmune diseases [129], allergies [130], tumors [131], transplants [132], and infections [133].
Interleukin 10 (IL-10)-producing B cells (B10 cells) are a subset of functional Bregs that inhibit experimental autoimmune encephalomyelitis, collagen-induced arthritis, and colitis inflammation [124,134,135]. B10 cells have been demonstrated to have strong potential in regulating inflammation in immune-mediated diseases and conditions in mice and humans [136,137,138]. Tedder et al. has studied the development, phenotype and effector function of mouse B10 cells, indicating that B10 cells are mainly concentrated in the spleen CD1dhi CD5+ B cell subsets [139]. In healthy individuals, B10 cells can inhibit the differentiation of naive T cells into pro-inflammatory T helper 1 (Th1) and Th2 subpopulations, and induce their differentiation into Tregs through IL-10 secretion [140].
Multiple TLR signalings have been implicated in the activation of B10 cells. Recent studies have demonstrated that IL-10 production and expansion of B10 cells were regulated by TLR2/4/9 signaling in vitro [141]. We investigated the B10 activation and expansion by TLR2/4/9 agonists (P. gingivalis LPS and CpG ODN) using isolated spleen B cells from P. gingivalis-immunized and non-immunized mice and the results indicated that P. gingivalis LPS and CpG differentially enhance IL-10 secretion and expansion of mouse B10 cells during innate and adaptive immune responses. CpG is a strong inducer of IL-10 expression in mouse B cells, and can further induce B10 cells to secrete IL-10 [136,142,143]. Studies have found that in these responses, CD1dhiCD5+ B cells are reduced and CpG enhances IL-10 capacity more effectively than P. gingivalis LPS [141]. Given the dual role of TLR signaling in the activation of both pro-inflammatory and anti-inflammatory pathways in the activated B cells, it is conceivable that promoting the optimal B10 function require selective activation of TLR signaling and availability of co-stimulatory molecules.
Studies have shown that multiple co-stimulatory molecules, such as IL-21, Tim-1, and CD40 signaling are involved in the functional maturation of B10 cells [144,145,146]. These data indicated that B10 cells are activated through multiple routes of action, enhanced by antigen specificity, and can be achieved independently of TLR ligation. Yang et al. demonstrated that the combination of IL-21, anti-Tim1 and CD40L can significantly induce the IL-10 potency of B10 cells in vitro and alleviate bone loss in experimental periodontitis in vivo [147]. Similarly, Yoshizaki et al. used a mouse model and showed the differentiation and maturation of B10 cells into functional effector cells that secrete IL-10 require IL-21 and CD40-dependent homologous interactions with T cells [148]. In addition, the stimulation of CD40 and IL-21 receptor signaling can drive the development and expansion of B10 cells by a few million times and generate B10 effector cells that secrete IL-10 in vitro [136]. Furthermore, Tim-1 is essential for the induction and maintenance of IL-10 in regulatory B cells and its regulatory function on inflammation [149,150]. Xiao et al. found that Tim-1-deficient B cells had reduced levels of IL-10 production, increased levels of pro-inflammatory cytokines, and enhanced Th1/Th17 responses [150]. Tim-1 expression was detected in more than 70% of IL-10-producing B cells, and co-stimulation of B cells with alloantigen and anti-Tim-1 antibody can increase B cell Tim-1, IL-10 and IL-4 expression, potentiating the regulatory function of B cells [151]. Activation of CD40 signaling seems to induce differentiation of B10 progenitor cell population and B10 cell maturation, leading to the cytoplasmic IL-10 expression during B10 cell development, but not inducing the secretion of IL-10 [136,152]. For a long time, it has been shown that the outcome of CD40–CD40L interaction may depend on the stage of B cell maturity and the duration or intensity of reciprocal signals between T cells and B cells. These signals may ultimately determine whether these B cells can differentiate into Bregs, memory cells or plasma cells [153,154]. It has also been suggested that CD40 signaling does not induce B cell clone expansion, but rather stimulates CD1dhi CD5+ B cells to become functionally more capable of producing IL-10 [152].
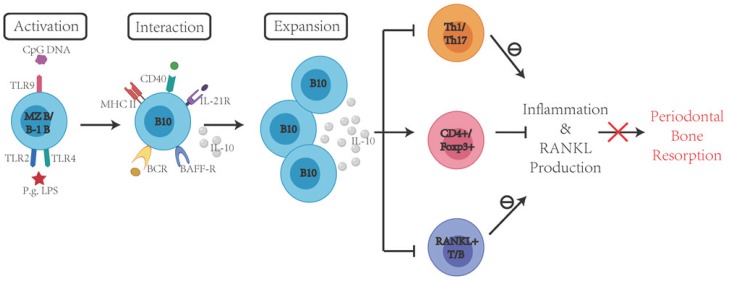
In vivo studies have shown that animals receiving adoptively transferred B10 cells demonstrated a reduced local inflammatory response [155], and that the local induction of IL-10 capacity of B10 cells is associated with the inhibition of inflammatory responses and periodontal bone loss in a murine model of ligature-induced experimental periodontitis in vivo [156]. Wang et al. demonstrated a significant increase in the percentage of B10 cells and the expression of IL-10 after combined treatment with P. gingivalis LPS (TLR2/4 agonist) and CpG (TLR9 agonist). For the first time, B10-rich CD1dhi CD5+ B cells were adoptively transferred to mice with experimental periodontitis and the findings demonstrated that B10-rich CD1dhi CD5+ B cells significantly inhibited RANKL production, periodontal inflammation and bone loss in a mouse model of experimental periodontitis, suggesting the regulatory role of B10 cells in the amelioration of periodontitis pathogenesis [155]. Shi et al. used purified antigen-specific B10 cells to adoptively transfer these cells into the recipient mice and demonstrated that B10 cells prevented inflammation-induced alveolar bone resorption by reducing periodontal osteoclastogenesis [157]. Moreover, compared with the control group, the mice receiving adoptive transfer of B10 cells showed reduced level of local RANKL production [157]. Evidence also indicates that adoptive transfer of B10 cells into animals can inhibit local IL-17 expression and effectively reduce the proportion of local Th17 cells [157]. It is suggested that B10 cells can regulate Treg/Th17 homeostasis during periodontitis and B10-Treg crosstalk may be involved in the regulation of local immune responses [157]. Indeed, animals with depleted B10 cells showed a significant lack of Treg cells, revealing the relationship between these two regulatory cell components [158]. Therefore, promoting B10 function locally can be a viable approach to rebalance immune response and ameliorate the progression of periodontal disease (Figure 3).
Figure 3.
Promoting local B10 function to curtail immune-mediated periodontal inflammation and bone loss. In periodontal disease, activated Th1 and Th17 cells produce pro-inflammatory cytokines that contribute to tissue damage. Both activated T and B cells produce RANKL, which leads to osteoclast activation and alveolar bone resorption. B10 cell activation and expansion promote IL-10 secretion and promote CD4+FOXP3+Treg activation, inhibit Th1/Th17 cells and RANKL+ T/B cells activation, which inhibit inflammation and RANKL production, eventually alleviating periodontal bone resorption.
Overall, a growing number of studies have demonstrated that Bregs are crucial in the maintenance of immune tolerance and in the suppression of inflammation. Several therapeutic interventions targeting Bregs have been proposed, including ex vivo expansion of Bregs, in vivo modulation to expand Bregs, and targeted depletion of specific Breg subsets, which could provide improved approaches for the treatment of immune-mediated diseases [159]. While significant progress has been made to understand the function of B10 cells, so far most of the research focused on its role on anti-inflammatory responses through IL-10 production. However, the function of B10 is not solely mediated by IL-10 production. Moreover, although experimental evidence has clearly demonstrated the role of B10 cells in the suppression of inflammatory responses, the description of multiple Breg cell subsets and the importance of inflammatory cytokines in the induction of Breg cells raised the possibility that the Breg ontogeny is associated with the interaction between multiple B cell subsets and other cells of the immune system in response to inflammation [160]. Studies have shown that Bregs regulate CD4+ T cells and promote Tregs through both cell–cell contact and cytokines [161], and Bregs inhibit CD8+ T cell proliferation through direct cognate interactions [162]. These findings suggest that cell–cell interaction is an essential component to execute Bregs functions.
Given the complex immune network with broad immune cell participation in chronic inflammation, the unidentified mechanism of the B10 regulatory role via cell–cell contact remains to be determined. Meanwhile, the question remains regarding the developmental link between Bregs and differentiated antibody-producing plasma cells [126]. More functional studies involving immune cell–cell interaction are needed to fully understand the mechanism of Bregs induction in vivo.
8. Concluding Remarks
Periodontal disease is initiated by oral dysbiosis, which lead to the immune and inflammatory responses that, on the one hand, control infection and protect the host from bacterial invasion, but on the other hand, cause collateral periodontal tissue destruction. This dual effect is reflected by the functional activities of immune cells and mediators involved in the pathogenesis of periodontitis. An increased understanding of the interactions between these cells of the immune system and the resident cells in periodontal tissue may allow us to identify new exciting targets for the treatment of periodontal disease. Among them, it appears that the activated T and B cells not only play a key role in controlling periodontal infections, but also act as a key factor in determining the extent of periodontal tissue destruction. Both human and experimental animal research support the hypothesis that by modulating the local host immune responses, there is considerable potential to interfere with periodontitis pathogenesis. Efforts are underway to evaluate strategies designed to interfere with the harmful effects of T and B cell activation to mitigate periodontal bone resorption. This may include reducing soluble RANKL activity or interfering with RANKL expression by T/B cells. Furthermore, as we gradually understand the key role of regulatory T and B cells in the control of periodontal disease progression, it will advance our understanding of the mechanisms underlying T/B cell-mediated immune regulation, and provide novel and effective targets of coordinated immune network that can be potentially modulated to achieve periodontal tissue homeostasis. Further insights into the mechanistic role of immune regulatory T and B cells in different disease settings will help us develop therapeutic strategies for better management of immune disorders.
Funding
This research was funded by National Institute of Dental and Craniofacial Research (NIDCR) grant number DE025255 to XH.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Herrera D., Retamal-Valdes B., Alonso B., Feres M. Acute periodontal lesions (periodontal abscesses and necrotizing periodontal diseases) and endo-periodontal lesions. J. Periodontol. 2018;89:S85–S102. doi: 10.1002/JPER.16-0642. [DOI] [PubMed] [Google Scholar]
- 2.Albandar J.M., Susin C., Hughes F.J. Manifestations of systemic diseases and conditions that affect the periodontal attachment apparatus: Case definitions and diagnostic considerations. J. Periodontol. 2018;89:S183–S203. doi: 10.1002/JPER.16-0480. [DOI] [PubMed] [Google Scholar]
- 3.Needleman I., Garcia R., Gkranias N., Kirkwood K.L., Kocher T., Iorio A.D., Moreno F., Petrie A. Mean annual attachment, bone level, and tooth loss: A systematic review. J. Periodontol. 2018;89:S120–S139. doi: 10.1002/JPER.17-0062. [DOI] [PubMed] [Google Scholar]
- 4.Billings M., Holtfreter B., Papapanou P.N., Mitnik G.L., Kocher T., Dye B.A. Age-dependent distribution of periodontitis in two countries: Findings from NHANES 2009 to 2014 and SHIP-TREND 2008 to 2012. J. Clin. Periodontol. 2018;45:S130–S148. doi: 10.1111/jcpe.12944. [DOI] [PubMed] [Google Scholar]
- 5.Papapanou P.N., Sanz M., Buduneli N., Dietrich T., Feres M., Fine D.H., Flemmig T.F., Garcia R., Giannobile W.V., Graziani F., et al. Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018;89:S173–S182. doi: 10.1002/JPER.17-0721. [DOI] [PubMed] [Google Scholar]
- 6.Fine D.H., Patil A.G., Loos B.G. Classification and diagnosis of aggressive periodontitis. J. Periodontol. 2018;89:S103–S119. doi: 10.1002/JPER.16-0712. [DOI] [PubMed] [Google Scholar]
- 7.Tonetti M.S., Greenwell H., Kornman K.S. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J. Periodontol. 2018;89:S159–S172. doi: 10.1002/JPER.18-0006. [DOI] [PubMed] [Google Scholar]
- 8.Mombelli A. Microbial colonization of the periodontal pocket and its significance for periodontal therapy. Periodontol. 2000. 2017;76:85–96. doi: 10.1111/prd.12147. [DOI] [PubMed] [Google Scholar]
- 9.Sochalska M., Potempa J. Manipulation of Neutrophils by Porphyromonas gingivalis in the Development of Periodontitis. Front. Microbiol. 2017;7:197. doi: 10.3389/fcimb.2017.00197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akrivopoulou C., Green I., Donos N., Nair S.P., Ready D. Aggregatibacter actinomycetemcomitans serotype prevalence and antibiotic resistance in a UK population with periodontitis. J. Glob. Antimicrob. Resist. 2017;10:54–58. doi: 10.1016/j.jgar.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 11.Mahalakshmi K., Krishnan P., Chandrasekaran S. Detection of Tannerella forsythia bspA and prtH genotypes among periodontitis patients and healthy subjects—A case—Control study. Arch. Oral Boil. 2018;96:178–181. doi: 10.1016/j.archoralbio.2018.09.012. [DOI] [PubMed] [Google Scholar]
- 12.De Andrade K.Q., Da Silva C.L.C.A., Coutinho-Silva R. Immunological Pathways Triggered byPorphyromonas gingivalisandFusobacterium nucleatum: Therapeutic Possibilities? Mediat. Inflamm. 2019;2019:7241312. doi: 10.1155/2019/7241312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kokubu E., Inoue T., Ishihara K. Response of epithelial cells infected by Treponema denticola. Oral Dis. 2018;24:14–18. doi: 10.1111/odi.12794. [DOI] [PubMed] [Google Scholar]
- 14.Willmann C., Mata X., Hanghøj K., Tonasso L., Tisseyre L., Jeziorski C., Cabot É., Chevet P., Crubézy É., Orlando L., et al. Oral health status in historic population: Macroscopic and metagenomic evidence. PLoS ONE. 2018;13:e0196482. doi: 10.1371/journal.pone.0196482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yumoto H., Hirota K., Hirao K., Miyazaki T., Yamamoto N., Miyamoto K., Murakami K., Fujiwara N., Matsuo T., Miyake Y. Anti-inflammatory and protective effects of 2-methacryloyloxyethyl phosphorylcholine polymer on oral epithelial cells. J. Biomed. Mater. Res. Part A. 2014;103:555–563. doi: 10.1002/jbm.a.35201. [DOI] [PubMed] [Google Scholar]
- 16.Fleetwood A.J., Lee M.K., Singleton W., Achuthan A., Lee M.-C., O’Brien-Simpson N.M., Cook A.D., Murphy A.J., Dashper S.G., Reynolds E.C., et al. Metabolic Remodeling, Inflammasome Activation, and Pyroptosis in Macrophages Stimulated by Porphyromonas gingivalis and Its Outer Membrane Vesicles. Front. Microbiol. 2017;7:351. doi: 10.3389/fcimb.2017.00351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gholizadeh P., Pormohammad A., Eslami H., Shokouhi B., Fakhrzadeh V., Kafil H.S. Oral pathogenesis of Aggregatibacter actinomycetemcomitans. Microb. Pathog. 2017;113:303–311. doi: 10.1016/j.micpath.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 18.Groeger S.E., Meyle J. Epithelial barrier and oral bacterial infection. Periodontol. 2000. 2015;69:46–67. doi: 10.1111/prd.12094. [DOI] [PubMed] [Google Scholar]
- 19.Cueno M.E., Ochiai K. Gingival Periodontal Disease (PD) Level-Butyric Acid Affects the Systemic Blood and Brain Organ: Insights Into the Systemic Inflammation of Periodontal Disease. Front. Immunol. 2018;9:1158. doi: 10.3389/fimmu.2018.01158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsai C.-C., Ho Y.-P., Chou Y.-S., Ho K.-Y., Wu Y.-M., Lin Y.-C. Aggregatibacter (Actinobacillus) actimycetemcomitans leukotoxin and human periodontitis—A historic review with emphasis on JP2. Kaohsiung J. Med. Sci. 2018;34:186–193. doi: 10.1016/j.kjms.2018.01.014. [DOI] [PubMed] [Google Scholar]
- 21.Greabu M., Totan A., Miricescu D., Radulescu R., Virlan J., Calenic B. Hydrogen Sulfide, Oxidative Stress and Periodontal Diseases: A Concise Review. Antioxidants. 2016;5:3. doi: 10.3390/antiox5010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meyle J., Dommisch H., Groeger S., Giacaman R.A., Costalonga M., Herzberg M. The innate host response in caries and periodontitis. J. Clin. Periodontol. 2017;44:1215–1225. doi: 10.1111/jcpe.12781. [DOI] [PubMed] [Google Scholar]
- 23.Taubman M.A., Yoshie H., Ebersole J., Smith D., Olson C. Host Response in Experimental Periodontal Disease. J. Dent. Res. 1984;63:455–460. doi: 10.1177/00220345840630031801. [DOI] [PubMed] [Google Scholar]
- 24.Pan W., Wang Q., Chen Q. The cytokine network involved in the host immune response to periodontitis. Int. J. Oral Sci. 2019;11:30. doi: 10.1038/s41368-019-0064-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taubman M.A., Valverde P., Han X., Kawai T. Immune Response: The Key to Bone Resorption in Periodontal Disease. J. Periodontol. 2005;76:2033–2041. doi: 10.1902/jop.2005.76.11-S.2033. [DOI] [PubMed] [Google Scholar]
- 26.Lundmark A., Hu Y.O.O., Huss M., Johannsen G., Andersson A.F., Yucel-Lindberg T. Identification of Salivary Microbiota and Its Association With Host Inflammatory Mediators in Periodontitis. Front. Microbiol. 2019;9:216. doi: 10.3389/fcimb.2019.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taubman M.A., Kawai T. Involvement of T-lymphocytes in periodontal disease and in direct and indirect induction of bone resorption. Crit. Rev. Oral Boil. Med. 2001;12:125–135. doi: 10.1177/10454411010120020301. [DOI] [PubMed] [Google Scholar]
- 28.Shaddox L.M., Spencer W.P., Velsko I.M., Al-Kassab H., Huang H., Calderon N., Aukhil I., Wallet S.M., Hiba H. Localized aggressive periodontitis immune response to healthy and diseased subgingival plaque. J. Clin. Periodontol. 2016;43:746–753. doi: 10.1111/jcpe.12560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Naruishi K., Nagata T. Biological effects of interleukin-6 on Gingival Fibroblasts: Cytokine regulation in periodontitis. J. Cell. Physiol. 2018;233:6393–6400. doi: 10.1002/jcp.26521. [DOI] [PubMed] [Google Scholar]
- 30.Cavalla F., Osorio C., Paredes R., Valenzuela M.A., Garcia-Sesnich J., Sorsa T., Tervahartiala T., Hernández M. Matrix metalloproteinases regulate extracellular levels of SDF-1/CXCL12, IL-6 and VEGF in hydrogen peroxide-stimulated human periodontal ligament fibroblasts. Cytokine. 2015;73:114–121. doi: 10.1016/j.cyto.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 31.Han M.-X., Ding C., Kyung H.M. Genetic polymorphisms in pattern recognition receptors and risk of periodontitis: Evidence based on 12,793 subjects. Hum. Immunol. 2015;76:496–504. doi: 10.1016/j.humimm.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 32.Kawai T., Akira S. TLR signaling. Cell Death Differ. 2006;13:816–825. doi: 10.1038/sj.cdd.4401850. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Y., Li X. Lipopolysaccharide-regulated production of bone sialoprotein and interleukin-8 in human periodontal ligament fibroblasts: The role of toll-like receptors 2 and 4 and the MAPK pathway. J. Periodontal Res. 2014;50:141–151. doi: 10.1111/jre.12193. [DOI] [PubMed] [Google Scholar]
- 34.Alqallaf H., Hamada Y., Blanchard S., Shin D., Gregory R., Srinivasan M. Differential profiles of soluble and cellular toll like receptor (TLR)-2 and 4 in chronic periodontitis. PLoS ONE. 2018;13:e0200231. doi: 10.1371/journal.pone.0200231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ilango P., Mahalingam A., Parthasarathy H., Katamreddy V., Subbareddy V. Evaluation of TLR2 and 4 in Chronic Periodontitis. J. Clin. Diagn. Res. 2016;10:ZC86–ZC89. doi: 10.7860/JCDR/2016/18353.8027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Babu S., Blauvelt C.P., Kumaraswami V., Nutman T.B. Cutting edge: Diminished T cell TLR expression and function modulates the immune response in human filarial infection. J. Immunol. 2006;176:3885–3889. doi: 10.4049/jimmunol.176.7.3885. [DOI] [PubMed] [Google Scholar]
- 37.Dorner M., Brandt S., Tinguely M., Zucol F., Bourquin J.-P., Zauner L., Berger C., Bernasconi M., Speck R.F., Nadal D. Plasma cell toll-like receptor (TLR) expression differs from that of B cells, and plasma cell TLR triggering enhances immunoglobulin production. Immunol. 2009;128:573–579. doi: 10.1111/j.1365-2567.2009.03143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zeng X., Zhang Y., Yang Q., Wang S., Zou B., Tan Y., Zou M., Liu S., Li X. Artesunate attenuates LPS-induced osteoclastogenesis by suppressing TLR4/TRAF6 and PLCγ1-Ca2+-NFATc1 signaling pathway. Acta Pharmacol. Sin. 2020;41:229–236. doi: 10.1038/s41401-019-0289-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li K., Lv G., Pan L. Sirt1 alleviates LPS induced inflammation of periodontal ligament fibroblasts via downregulation of TLR4. Int. J. Boil. Macromol. 2018;119:249–254. doi: 10.1016/j.ijbiomac.2018.07.099. [DOI] [PubMed] [Google Scholar]
- 40.Zhang J., Yu C., Zhang X., Chen H., Dong J.-C., Lu W., Song Z.-C., Zhou W. Porphyromonas gingivalis lipopolysaccharide induces cognitive dysfunction, mediated by neuronal inflammation via activation of the TLR4 signaling pathway in C57BL/6 mice. J. Neuroinflammation. 2018;15:37. doi: 10.1186/s12974-017-1052-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ding P.-H., Darveau R.P., Wang C.-Y., Jin L. 3LPS-binding protein and its interactions with P. gingivalis LPS modulate pro-inflammatory response and Toll-like receptor signaling in human oral keratinocytes. PLoS ONE. 2017;12:e0173223. doi: 10.1371/journal.pone.0173223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chukkapalli S.S., Velsko I.M., Rivera-Kweh M.F., Larjava H., Lucas A., Kesavalu L. Global TLR2 and 4 deficiency in mice impacts bone resorption, inflammatory markers and atherosclerosis to polymicrobial infection. Mol. Oral Microbiol. 2016;32:211–225. doi: 10.1111/omi.12165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hajishengallis G., Sojar H., Genco R.J., DeNardin E. Intracellular Signaling and Cytokine Induction upon Interactions ofPorphyromonas gingivalisFimbriae with Pattern-Recognition Receptors. Immunol. Investig. 2004;33:157–172. doi: 10.1081/IMM-120030917. [DOI] [PubMed] [Google Scholar]
- 44.Kim P.D., Xia-Juan X., Crump K.E., Abe T., Hajishengallis G., Sahingur S.E. Toll-Like Receptor 9-Mediated Inflammation Triggers Alveolar Bone Loss in Experimental Murine Periodontitis. Infect. Immun. 2015;83:2992–3002. doi: 10.1128/IAI.00424-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Song B., Zhang Y., Chen L., Zhou T., Huang W., Zhou X., Shao L., Zhang Y., Chen L., Huang W., et al. The role of Toll-like receptors in periodontitis. Oral Dis. 2016;23:168–180. doi: 10.1111/odi.12468. [DOI] [PubMed] [Google Scholar]
- 46.Crump K.E., Oakley J.C., Xia-Juan X., Madu T.C., Devaki S., Mooney E.C., Sahingur S.E. Interplay of Toll-Like Receptor 9, Myeloid Cells, and Deubiquitinase A20 in Periodontal Inflammation. Infect. Immun. 2016;85:e00814-16. doi: 10.1128/IAI.00814-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Souto G.R., Queiroz C.M., Costa F.O., Mesquita R.A. Relationship Between Chemokines and Dendritic Cells in Human Chronic Periodontitis. J. Periodontol. 2014;85:1416–1423. doi: 10.1902/jop.2014.130662. [DOI] [PubMed] [Google Scholar]
- 48.Cavalla F., Araujo-Pires A.C., Biguetti C.C., Garlet G.P. Cytokine Networks Regulating Inflammation and Immune Defense in the Oral Cavity. Curr. Oral Health Rep. 2014;1:104–113. doi: 10.1007/s40496-014-0016-9. [DOI] [Google Scholar]
- 49.Fiorillo L., Cervino G., Herford A., Lauritano F., D’Amico C., Giudice R.L., Laino L., Troiano G., Crimi S., Cicciù M. Interferon Crevicular Fluid Profile and Correlation with Periodontal Disease and Wound Healing: A Systemic Review of Recent Data. Int. J. Mol. Sci. 2018;19:1908. doi: 10.3390/ijms19071908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Silva N., Abusleme L., Bravo D., Dutzan N., Garcia-Sesnich J., Vernal R., Hernández M., Gamonal J. Host response mechanisms in periodontal diseases. J. Appl. Oral Sci. 2015;23:329–355. doi: 10.1590/1678-775720140259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boelen G.-J., Boute L., D’Hoop J., Ezeldeen M., Lambrichts I., Opdenakker G. Matrix metalloproteinases and inhibitors in dentistry. Clin. Oral Investig. 2019;23:2823–2835. doi: 10.1007/s00784-019-02915-y. [DOI] [PubMed] [Google Scholar]
- 52.Kang W., Hu Z., Ge S. Healthy and Inflamed Gingival Fibroblasts Differ in Their Inflammatory Response to Porphyromonas gingivalis Lipopolysaccharide. Inflammation. 2016;39:1842–1852. doi: 10.1007/s10753-016-0421-4. [DOI] [PubMed] [Google Scholar]
- 53.Ohgi K., Kajiya H., Goto-T K., Okamoto F., Yoshinaga Y., Okabe K., Sakagami R. Toll-like receptor 2 activation primes and upregulates osteoclastogenesis via lox-1. Lipids Health Dis. 2018;17:132. doi: 10.1186/s12944-018-0787-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kishimoto T., Kaneko T., Ukai T., Yokoyama M., Haro E.A., Yoshinaga Y., Yoshimura A., Hara Y. Peptidoglycan and lipopolysaccharide synergistically enhance bone resorption and osteoclastogenesis. J. Periodontal Res. 2012;47:446–454. doi: 10.1111/j.1600-0765.2011.01452.x. [DOI] [PubMed] [Google Scholar]
- 55.Zhang P., Liu J., Xu Q., Harber G., Feng X., Michalek S.M., Katz J. TLR2-dependent modulation of osteoclastogenesis by Porphyromonas gingivalis through differential induction of NFATc1 and NF-kappaB. J. Biol. Chem. 2011;286:24159–24169. doi: 10.1074/jbc.M110.198085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yu X., Hu Y., Freire M., Yu P., Kawai T., Han X. Role of toll-like receptor 2 in inflammation and alveolar bone loss in experimental peri-implantitis versus periodontitis. J. Periodontal Res. 2017;53:98–106. doi: 10.1111/jre.12492. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 57.Tsukasaki M., Takayanagi H. Osteoimmunology: Evolving concepts in bone–immune interactions in health and disease. Nat. Rev. Immunol. 2019;19:626–642. doi: 10.1038/s41577-019-0178-8. [DOI] [PubMed] [Google Scholar]
- 58.Lin X., Han X., Kawai T., Taubman M.A. Antibody to Receptor Activator of NF-κB Ligand Ameliorates T Cell-Mediated Periodontal Bone Resorption ▿. Infect. Immun. 2010;79:911–917. doi: 10.1128/IAI.00944-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gruber R. Osteoimmunology: Inflammatory osteolysis and regeneration of the alveolar bone. J. Clin. Periodontol. 2019;46:52–69. doi: 10.1111/jcpe.13056. [DOI] [PubMed] [Google Scholar]
- 60.Han X., Kawai T., Taubman M.A. Interference with immune-cell-mediated bone resorption in periodontal disease. Periodontol. 2000. 2007;45:76–94. doi: 10.1111/j.1600-0757.2007.00215.x. [DOI] [PubMed] [Google Scholar]
- 61.Li S., Hao L., Wang L., Lu Y., Li Q., Zhu Z., Shao J.-Z., Chen W. Targeting Atp6v1c1 Prevents Inflammation and Bone Erosion Caused by Periodontitis and Reveals Its Critical Function in Osteoimmunology. PLoS ONE. 2015;10:e0134903. doi: 10.1371/journal.pone.0134903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Parente R., Sobacchi C., Bottazzi B., Mantovani A., Grčevic D., Inforzato A. The Long Pentraxin PTX3 in Bone Homeostasis and Pathology. Front. Immunol. 2019;10:2628. doi: 10.3389/fimmu.2019.02628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sima C., Viniegra A., Glogauer M. Macrophage immunomodulation in chronic osteolytic diseases-the case of periodontitis. J. Leukoc. Boil. 2018;105:473–487. doi: 10.1002/JLB.1RU0818-310R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Taubman M.A., Kawai T., Han X. The new concept of periodontal disease pathogenesis requires new and novel therapeutic strategies. J. Clin. Periodontol. 2007;34:367–369. doi: 10.1111/j.1600-051X.2007.01065.x. [DOI] [PubMed] [Google Scholar]
- 65.Cardoso E.M., Arosa F. CD8+ T Cells in Chronic Periodontitis: Roles and Rules. Front. Immunol. 2017;8:30. doi: 10.3389/fimmu.2017.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Teng Y.-T.A. The role of acquired immunity and periodontal disease progression. Crit. Rev. Oral Boil. Med. 2003;14:237–252. doi: 10.1177/154411130301400402. [DOI] [PubMed] [Google Scholar]
- 67.Han X., Lin X., Yu X., Lin J., Kawai T., LaRosa K.B., Taubman M.A. Porphyromonas gingivalis Infection-Associated Periodontal Bone Resorption Is Dependent on Receptor Activator of NF-κB Ligand. Infect. Immun. 2013;81:1502–1509. doi: 10.1128/IAI.00043-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tsukasaki M., Komatsu N., Nagashima K., Nitta T., Pluemsakunthai W., Shukunami C., Iwakura Y., Nakashima T., Okamoto K., Takayanagi H. Host defense against oral microbiota by bone-damaging T cells. Nat. Commun. 2018;9:701. doi: 10.1038/s41467-018-03147-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yoshie H., Taubman M.A., Olson C.L., Ebersole J.L., Smith D.J., Yoshie H. Periodontal bone loss and immune characteristics after adoptive transfer of Actinobacilus-sensitized T cells to rats. J. Periodontal Res. 1987;22:499–505. doi: 10.1111/j.1600-0765.1987.tb02061.x. [DOI] [PubMed] [Google Scholar]
- 70.Baker P.J., Garneau J., Howe L., Roopenian D.C. T-cell contributions to alveolar bone loss in response to oral infection withPorphyromonas gingivalis. Acta Odontol. Scand. 2001;59:222–225. doi: 10.1080/00016350152509247. [DOI] [PubMed] [Google Scholar]
- 71.Kawai T., Shimauchi H., Eastcott J.W., Smith D.J., Taubman M.A. Antigen direction of specific T-cell clones into gingival tissues. Immunology. 1998;93:11–19. doi: 10.1046/j.1365-2567.1998.00408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Najafian N., Sayegh M.H. CTLA4-Ig: A novel immunosuppressive agent. Expert Opin. Investig. Drugs. 2000;9:2147–2157. doi: 10.1517/13543784.9.9.2147. [DOI] [PubMed] [Google Scholar]
- 73.Gemmell E., McHugh G.B., Grieco D.A., Seymour G. Costimulatory molecules in human periodontal disease tissues. J. Periodontal Res. 2001;36:92–100. doi: 10.1034/j.1600-0765.2001.360205.x. [DOI] [PubMed] [Google Scholar]
- 74.Yamashita K., Eastcott J.W., Taubman M.A., Smith D.J., Cox D.S. Effect of adoptive transfer of cloned Actinobacillus actinomycetemcomitans-specific T helper cells on periodontal disease. Infect. Immun. 1991;59:1529–1534. doi: 10.1128/IAI.59.4.1529-1534.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Eastcott J.W., Yamashita K., Taubman M.A., Smith D.J. Characterization of rat T-cell clones with bacterial specificity. Immunology. 1990;71:120–126. [PMC free article] [PubMed] [Google Scholar]
- 76.Weitzmann M.N. Bone and the Immune System. Toxicol. Pathol. 2017;45:911–924. doi: 10.1177/0192623317735316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kozuka Y., Ozaki Y., Ukai T., Kaneko T., Hara Y. B Cells Play an Important Role in Lipopolysaccharide-Induced Bone Resorption. Calcif. Tissue Int. 2006;78:125–132. doi: 10.1007/s00223-005-0149-x. [DOI] [PubMed] [Google Scholar]
- 78.Han X., Kawai T., Eastcott J.W., Taubman M.A. Bacterial-responsive B lymphocytes induce periodontal bone resorption. J. Immunol. 2006;176:625–631. doi: 10.4049/jimmunol.176.1.625. [DOI] [PubMed] [Google Scholar]
- 79.Harada Y., Han X., Yamashita K., Kawai T., Eastcott J.W., Smith D.J., Taubman M.A. Effect of adoptive transfer of antigen-specific B cells on periodontal bone resorption. J. Periodontal Res. 2006;41:101–107. doi: 10.1111/j.1600-0765.2005.00839.x. [DOI] [PubMed] [Google Scholar]
- 80.Kawai T., Eisen-Lev R., Seki M., Eastcott J.W., Wilson M.E., Taubman M.A. Requirement of B7 costimulation for Th1-mediated inflammatory bone resorption in experimental periodontal disease. J. Immunol. 2000;164:2102–2109. doi: 10.4049/jimmunol.164.4.2102. [DOI] [PubMed] [Google Scholar]
- 81.Kenkre J., Bassett J.H.D. The bone remodelling cycle. Ann. Clin. Biochem. Int. J. Lab. Med. 2018;55:308–327. doi: 10.1177/0004563218759371. [DOI] [PubMed] [Google Scholar]
- 82.Martin T.J., Sims N. RANKL/OPG; Critical role in bone physiology. Rev. Endocr. Metab. Disord. 2015;16:131–139. doi: 10.1007/s11154-014-9308-6. [DOI] [PubMed] [Google Scholar]
- 83.Han S.-Y., Kim Y.-K. Berberine Suppresses RANKL-Induced Osteoclast Differentiation by Inhibiting c-Fos and NFATc1 Expression. Am. J. Chin. Med. 2019;47:439–455. doi: 10.1142/S0192415X19500228. [DOI] [PubMed] [Google Scholar]
- 84.Kanzaki H., Makihira S., Suzuki M., Ishii T., Movila A., Hirschfeld J., Mawardi H., Lin X., Han X., Taubman M.A., et al. Soluble RANKL Cleaved from Activated Lymphocytes by TNF-α-Converting Enzyme Contributes to Osteoclastogenesis in Periodontitis. J. Immunol. 2016;197:3871–3883. doi: 10.4049/jimmunol.1601114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mizuno A., Kanno T., Hoshi M., Shibata O., Yano K., Fujise N., Kinosaki M., Yamaguchi K., Tsuda E., Murakami A., et al. Transgenic mice overexpressing soluble osteoclast differentiation factor (sODF) exhibit severe osteoporosis. J. Bone Miner. Metab. 2002;20:337–344. doi: 10.1007/s007740200049. [DOI] [PubMed] [Google Scholar]
- 86.Niu C., Xiao F., Yuan K., Hu X., Lin W., Ma R., Zhang X., Huang Z. Nardosinone Suppresses RANKL-Induced Osteoclastogenesis and Attenuates Lipopolysaccharide-Induced Alveolar Bone Resorption. Front. Pharmacol. 2017;8:626. doi: 10.3389/fphar.2017.00626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kuritani M., Sakai N., Karakawa A., Isawa M., Chatani M., Negishi-Koga T., Funatsu T., Takami M. Anti-mouse RANKL Antibodies Inhibit Alveolar Bone Destruction in Periodontitis Model Mice. Boil. Pharm. Bull. 2018;41:637–643. doi: 10.1248/bpb.b18-00026. [DOI] [PubMed] [Google Scholar]
- 88.Degertekin C.K., Turhan Iyidir O., Yılmaz B.A., Elbeg S., Pasaoglu O.T., Pasaoglu H., Cakır N., Arslan M. RANKL/Osteoprotegerin System and Bone Turnover in Hashimoto Thyroiditis. Calcif. Tissue Int. 2016;99:365–372. doi: 10.1007/s00223-016-0163-1. [DOI] [PubMed] [Google Scholar]
- 89.Ferreira E.C., Bortolin R.H., Freire-Neto F.P., Souza K.S., Bezerra J., Ururahy M.A., Ramos A.M.D.O., Himelfarb S.T., Abreu B.J., DiDone T.V., et al. Zinc supplementation reduces RANKL/OPG ratio and prevents bone architecture alterations in ovariectomized and type 1 diabetic rats. Nutr. Res. 2017;40:48–56. doi: 10.1016/j.nutres.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 90.El Kholy K., Freire M., Chen T., Van Dyke T.E. Resolvin E1 Promotes Bone Preservation Under Inflammatory Conditions. Front. Immunol. 2018;9:1300. doi: 10.3389/fimmu.2018.01300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Costa L.C., Da Fonseca M.A., Pinheiro A.D.R., Aguiar T.R.D.S., Machado A.N., Quinelato V., Bonato L.L., Aguiar D.P., Vieira T., De Almeida F.L.D., et al. Chronic Periodontitis and RANKL/OPG Ratio in Peri-Implant Mucosae Inflammation. Braz. Dent. J. 2018;29:14–22. doi: 10.1590/0103-6440201801241. [DOI] [PubMed] [Google Scholar]
- 92.Salinas-Muñoz M., Garrido-Flores M., Baeza M., Huaman P., García-Sesnich J., Bologna R., Vernal R., Hernández M. Bone resorptive activity in symptomatic and asymptomatic apical lesions of endodontic origin. Clin. Oral Investig. 2017;21:2613–2618. doi: 10.1007/s00784-017-2062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.De Rossi A., Fukada S.Y., De Rossi M., Da Silva R.A.B., Queiroz A.M., Nelson-Filho P., Da Silva L.A.B. Cementocytes Express Receptor Activator of the Nuclear Factor Kappa-B Ligand in Response to Endodontic Infection in Mice. J. Endod. 2016;42:1251–1257. doi: 10.1016/j.joen.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 94.Rajendran M., Looney S., Singh N., Elashiry M., Meghil M.M., El-Awady A.R., Tawfik O., Susin C., Arce R.M., Cutler C.W. Systemic Antibiotic Therapy Reduces Circulating Inflammatory Dendritic Cells and Treg-Th17 Plasticity in Periodontitis. J. Immunol. 2019;202:2690–2699. doi: 10.4049/jimmunol.1900046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Braz-Silva P.H., Bergamini M.L., Mardegan A.P., De Rosa C.S., Hasseus B., Jonasson P. Inflammatory profile of chronic apical periodontitis: A literature review. Acta Odontol. Scand. 2018;77:173–180. doi: 10.1080/00016357.2018.1521005. [DOI] [PubMed] [Google Scholar]
- 96.Yuce H.B., Gokturk O., Turkal H.A., Inanir A., Benli I., Demir O. Assessment of local and systemic 25-hydroxy-vitamin D, RANKL, OPG, and TNF levels in patients with rheumatoid arthritis and periodontitis. J. Oral Sci. 2017;59:397–404. doi: 10.2334/josnusd.16-0677. [DOI] [PubMed] [Google Scholar]
- 97.Figueredo C.M.D.S., Lira-Junior R., Love R. T and B Cells in Periodontal Disease: New Functions in A Complex Scenario. Int. J. Mol. Sci. 2019;20:3949. doi: 10.3390/ijms20163949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Melgar-Rodríguez S., Díaz-Zúñiga J., Alvarez C., Rojas L., Monasterio G., Carvajal P., Escobar A., Sanz M., Vernal R. Serotype b ofAggregatibacter actinomycetemcomitansincreases osteoclast and memory T-lymphocyte activation. Mol. Oral Microbiol. 2015;31:162–174. doi: 10.1111/omi.12112. [DOI] [PubMed] [Google Scholar]
- 99.Palioto D.B., Finoti L.S., Kinane D.F., Benakanakere M. Epigenetic and inflammatory events in experimental periodontitis following systemic microbial challenge. J. Clin. Periodontol. 2019;46:819–829. doi: 10.1111/jcpe.13151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chen B., Wu W., Sun W., Zhang Q., Yan F., Xiao Y. RANKL Expression in Periodontal Disease: Where Does RANKL Come from? BioMed Res. Int. 2014;2014:731039. doi: 10.1155/2014/731039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Han Y., Jin Y., Miao Y., Shi T., Lin X. Improved RANKL production by memory B cells: A way for B cells promote alveolar bone destruction during periodontitis. Int. Immunopharmacol. 2018;64:232–237. doi: 10.1016/j.intimp.2018.08.033. [DOI] [PubMed] [Google Scholar]
- 102.Han Y., Jin Y., Miao Y., Shi T., Lin X. Improved RANKL expression and osteoclastogenesis induction of CD27+CD38− memory B cells: A link between B cells and alveolar bone damage in periodontitis. J. Periodontal Res. 2019;54:73–80. doi: 10.1111/jre.12606. [DOI] [PubMed] [Google Scholar]
- 103.Kawai T., Matsuyama T., Hosokawa Y., Makihira S., Seki M., Karimbux N.Y., Gonçalves R.B., Valverde P., Dibart S., Li Y.-P., et al. B and T Lymphocytes Are the Primary Sources of RANKL in the Bone Resorptive Lesion of Periodontal Disease. Am. J. Pathol. 2006;169:987–998. doi: 10.2353/ajpath.2006.060180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Miyazaki Y., Nakayamada S., Kubo S., Nakano K., Iwata S., Miyagawa I., Ma X., Trimova G., Sakata K., Tanaka Y. Th22 Cells Promote Osteoclast Differentiation via Production of IL-22 in Rheumatoid Arthritis. Front. Immunol. 2018;9:2901. doi: 10.3389/fimmu.2018.02901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Manabe N., Kawaguchi H., Chikuda H., Miyaura C., Inada M., Nagai R., Nabeshima Y.-I., Nakamura K., Sinclair A.M., Scheuermann R.H., et al. Connection between B lymphocyte and osteoclast differentiation pathways. J. Immunol. 2001;167:2625–2631. doi: 10.4049/jimmunol.167.5.2625. [DOI] [PubMed] [Google Scholar]
- 106.Teng Y.-T.A., Nguyen H., Gao X., Kong Y.-Y., Gorczynski R.M., Singh B., Ellen R.P., Penninger J.M. Functional human T-cell immunity and osteoprotegerin ligand control alveolar bone destruction in periodontal infection. J. Clin. Investig. 2000;106:R59–R67. doi: 10.1172/JCI10763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kawai T., Paster B.J., Komatsuzawa H., Ernst C.W.O., Goncalves R.B., Sasaki H., Ouhara K., Stashenko P.P., Sugai M., Taubman M.A. Cross-reactive adaptive immune response to oral commensal bacteria results in an induction of receptor activator of nuclear factor-?B ligand (RANKL)-dependent periodontal bone resorption in a mouse model. Oral Microbiol. Immunol. 2007;22:208–215. doi: 10.1111/j.1399-302X.2007.00348.x. [DOI] [PubMed] [Google Scholar]
- 108.Crotti T., Smith M.D., Hirsch R., Soukoulis S., Weedon H., Capone M., Ahern M.J., Haynes D. Receptor activator NF κB ligand (RANKL) and osteoprotegerin (OPG) protein expression in periodontitis. J. Periodontal Res. 2003;38:380–387. doi: 10.1034/j.1600-0765.2003.00615.x. [DOI] [PubMed] [Google Scholar]
- 109.Jin Q., Cirelli J.A., Park C.H., Sugai J.V., Taba M., Kostenuik P.J., Giannobile W. RANKL inhibition through osteoprotegerin blocks bone loss in experimental periodontitis. J. Periodontol. 2007;78:1300–1308. doi: 10.1902/jop.2007.070073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kassem A., Henning P., Lundberg P., De Souza P.P.C., Lindholm C., Lerner U.H. Porphyromonas gingivalis Stimulates Bone Resorption by Enhancing RANKL (Receptor Activator of NF-κB Ligand) through Activation of Toll-like Receptor 2 in Osteoblasts*. J. Boil. Chem. 2015;290:20147–20158. doi: 10.1074/jbc.M115.655787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Amcheslavsky A., Bar-Shavit Z. Interleukin (IL)-12 mediates the anti-osteoclastogenic activity of CpG-oligodeoxynucleotides. J. Cell. Physiol. 2006;207:244–250. doi: 10.1002/jcp.20563. [DOI] [PubMed] [Google Scholar]
- 112.Ji J.-D., Park-Min K.-H., Shen Z., Fajardo R.J., Goldring S.R., McHugh K.P., Ivashkiv L.B. Inhibition of RANK expression and osteoclastogenesis by TLRs and IFN-gamma in human osteoclast precursors. J. Immunol. 2009;183:7223–7233. doi: 10.4049/jimmunol.0900072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yu X., Lin J., Yu Q., Kawai T., Taubman M.A., Han X. Activation of Toll-like receptor 9 inhibits lipopolysaccharide-induced receptor activator of nuclear factor kappa- B ligand expression in rat B lymphocytes. Microbiol. Immunol. 2014;58:51–60. doi: 10.1111/1348-0421.12115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lin M., Hu Y., Wang Y., Kawai T., Wang Z., Han X. Different engagement of TLR2 and TLR4 in Porphyromonas gingivalis vs. ligature-induced periodontal bone loss. Braz. Oral Res. 2017;31:e63. doi: 10.1590/1807-3107bor-2017.vol31.0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.AlQranei M.S., Chellaiah M.A. Osteoclastogenesis in periodontal diseases: Possible mediators and mechanisms. J. Oral Biosci. 2020 doi: 10.1016/j.job.2020.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Garlet G.P., Sfeir C.S., Little S.R. Restoring host-microbe homeostasis via selective chemoattraction of Tregs. J. Dent. Res. 2014;93:834–839. doi: 10.1177/0022034514544300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Garlet G.P., Cardoso C.R.D.B., Mariano F.S., Claudino M., Assis G.F., Campanelli A.P., Avila-Campos M., Silva J.S. Regulatory T cells attenuate experimental periodontitis progression in mice. J. Clin. Periodontol. 2009;37:591–600. doi: 10.1111/j.1600-051X.2010.01586.x. [DOI] [PubMed] [Google Scholar]
- 118.Glowacki A.J., Gottardi R., Yoshizawa S., Cavalla F., Garlet G.P., Sfeir C., Little S.R. Strategies to direct the enrichment, expansion, and recruitment of regulatory cells for the treatment of disease. Ann. Biomed. Eng. 2014;43:593–602. doi: 10.1007/s10439-014-1125-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zouali M. The emerging roles of B cells as partners and targets in periodontitis. Autoimmunity. 2016;50:61–70. doi: 10.1080/08916934.2016.1261841. [DOI] [PubMed] [Google Scholar]
- 120.Astashina N., Sergeeva E.S., IG. Lukanin A.N., Kazakov S.V. Evaluating the effectiveness of the new mouthguard design in athletes involved in power sports. Stomatologiia. 2016;95:40–43. doi: 10.17116/stomat201695640-43. [DOI] [PubMed] [Google Scholar]
- 121.Dutzan N., Konkel J.E., Greenwell-Wild T., Moutsopoulos N.M. Characterization of the human immune cell network at the gingival barrier. Mucosal Immunol. 2016;9:1163–1172. doi: 10.1038/mi.2015.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wolf S.D., Dittel B.N., Hardardottir F., Janeway C.A. Experimental Autoimmune Encephalomyelitis Induction in Genetically B Cell–deficient Mice. J. Exp. Med. 1996;184:2271–2278. doi: 10.1084/jem.184.6.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mizoguchi A., Mizoguchi E., Smith R.N., Preffer F.I., Bhan A.K. Suppressive Role of B Cells in Chronic Colitis of T Cell Receptor α Mutant Mice. J. Exp. Med. 1997;186:1749–1756. doi: 10.1084/jem.186.10.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mizoguchi A., Mizoguchi E., Takedatsu H., Blumberg R.S., Bhan A.K. Chronic intestinal inflammatory condition generates IL-10-producing regulatory B cell subset characterized by CD1d upregulation. Immunity. 2002;16:219–230. doi: 10.1016/S1074-7613(02)00274-1. [DOI] [PubMed] [Google Scholar]
- 125.Mauri C., Bosma A. Immune Regulatory Function of B Cells. Annu. Rev. Immunol. 2012;30:221–241. doi: 10.1146/annurev-immunol-020711-074934. [DOI] [PubMed] [Google Scholar]
- 126.Fillatreau S. Natural regulatory plasma cells. Curr. Opin. Immunol. 2018;55:62–66. doi: 10.1016/j.coi.2018.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Baba Y., Matsumoto M., Kurosaki T. Signals controlling the development and activity of regulatory B-lineage cells. Int. Immunol. 2015;27:487–493. doi: 10.1093/intimm/dxv027. [DOI] [PubMed] [Google Scholar]
- 128.Shen P., Fillatreau S. Antibody-independent functions of B cells: A focus on cytokines. Nat. Rev. Immunol. 2015;15:441–451. doi: 10.1038/nri3857. [DOI] [PubMed] [Google Scholar]
- 129.Ray A., Dittel B.N. Mechanisms of Regulatory B cell Function in Autoimmune and Inflammatory Diseases beyond IL-10. J. Clin. Med. 2017;6:12. doi: 10.3390/jcm6010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Braza F., Chesné J., Castagnet S., Magnan A., Brouard S. Regulatory functions of B cells in allergic diseases. Allergy. 2014;69:1454–1463. doi: 10.1111/all.12490. [DOI] [PubMed] [Google Scholar]
- 131.Zhang Y., Gallastegui N., Rosenblatt J.D. Regulatory B cells in anti-tumor immunity. Int. Immunol. 2015;27:521–530. doi: 10.1093/intimm/dxv034. [DOI] [PubMed] [Google Scholar]
- 132.Wortel C., Heidt S. Regulatory B cells: Phenotype, function and role in transplantation. Transpl. Immunol. 2017;41:1–9. doi: 10.1016/j.trim.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 133.Shen P., Fillatreau S. Suppressive functions of B cells in infectious diseases: Fig. 1. Int. Immunol. 2015;27:513–519. doi: 10.1093/intimm/dxv037. [DOI] [PubMed] [Google Scholar]
- 134.Mauri C., Gray D., Mushtaq N., Londei M. Prevention of Arthritis by Interleukin 10–producing B Cells. J. Exp. Med. 2003;197:489–501. doi: 10.1084/jem.20021293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Fillatreau S., Sweenie C.H., McGeachy M.J., Gray D., Anderton S.M. B cells regulate autoimmunity by provision of IL-10. Nat. Immunol. 2002;3:944–950. doi: 10.1038/ni833. [DOI] [PubMed] [Google Scholar]
- 136.Yanaba K., Bouaziz J.-D., Matsushita T., Tsubata T., Tedder T.F. The development and function of regulatory B cells expressing IL-10 (B10 cells) requires antigen receptor diversity and TLR signals. J. Immunol. 2009;182:7459–7472. doi: 10.4049/jimmunol.0900270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lin M., Lin J., Wang Y., Bonheur N., Kawai T., Wang Z., Han X. Lipopolysaccharide Attenuates CD40 Ligand-Induced Regulatory B10 Cell Expansion and IL-10 Production in Mouse Splenocytes. Open J. Immunol. 2015;5:1–8. doi: 10.4236/oji.2015.51001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lykken J.M., Candando K.M., Tedder T.F. Regulatory B10 cell development and function. Int. Immunol. 2015;27:471–477. doi: 10.1093/intimm/dxv046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Tedder T.F. B10 Cells: A Functionally Defined Regulatory B Cell Subset. J. Immunol. 2015;194:1395–1401. doi: 10.4049/jimmunol.1401329. [DOI] [PubMed] [Google Scholar]
- 140.Flores-Borja F., Bosma A., Ng D., Reddy V., Ehrenstein M.R., Isenberg D.A., Mauri C. CD19+CD24hiCD38hi B Cells Maintain Regulatory T Cells While Limiting TH1 and TH17 Differentiation. Sci. Transl. Med. 2013;5:173ra23. doi: 10.1126/scitranslmed.3005407. [DOI] [PubMed] [Google Scholar]
- 141.Liu Z., Hu Y., Yu P., Lin M., Huang G., Kawai T., Taubman M., Wang Z., Xiaozhe H. Toll-like receptor agonists Porphyromonas gingivalis LPS and CpG differentially regulate IL-10 competency and frequencies of mouse B10 cells. J. Appl. Oral Sci. 2017;25:90–100. doi: 10.1590/1678-77572016-0277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kalampokis I., Yoshizaki A., Tedder T.F. IL-10-producing regulatory B cells (B10 cells) in autoimmune disease. Arthritis Res. 2013;15:S1. doi: 10.1186/ar3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Barr T.A., Brown S., Ryan G., Zhao J., Gray D. TLR-mediated stimulation of APC: Distinct cytokine responses of B cells and dendritic cells. Eur. J. Immunol. 2007;37:3040–3053. doi: 10.1002/eji.200636483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Leavy O. B cells: IL-21 promotes B10 cell population expansion. Nat. Rev. Immunol. 2012;12:808–809. doi: 10.1038/nri3346. [DOI] [PubMed] [Google Scholar]
- 145.Yeung M.Y., Ding Q., Brooks C.R., Xiao S., Workman C.J., Vignali D.A., Ueno T., Padera R.F., Kuchroo V.K., Najafian N., et al. TIM-1 signaling is required for maintenance and induction of regulatory B cells. Arab. Archaeol. Epigr. 2015;15:942–953. doi: 10.1111/ajt.13087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Poe J.C., Smith S.H., Haas K.M., Yanaba K., Tsubata T., Matsushita T., Tedder T.F. Amplified B Lymphocyte CD40 Signaling Drives Regulatory B10 Cell Expansion in Mice. PLoS ONE. 2011;6:22464. doi: 10.1371/journal.pone.0022464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hu Y., Yu P., Yu X., Hu X., Kawai T., Han X. IL-21/anti-Tim1/CD40 ligand promotes B10 activity in vitro and alleviates bone loss in experimental periodontitis in vivo. Biochim. Biophys. Acta Mol. Basis Dis. 2017;1863:2149–2157. doi: 10.1016/j.bbadis.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Yoshizaki A., Miyagaki T., DiLillo D.J., Matsushita T., Horikawa M., Kountikov E.I., Spolski R., Poe J.C., Leonard W.J., Tedder T.F. Regulatory B cells control T-cell autoimmunity through IL-21-dependent cognate interactions. Nature. 2012;491:264–268. doi: 10.1038/nature11501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ma J., Usui Y., Takeda K., Harada N., Yagita H., Okumura K., Akiba H. TIM-1 signaling in B cells regulates antibody production. Biochem. Biophys. Res. Commun. 2011;406:223–228. doi: 10.1016/j.bbrc.2011.02.021. [DOI] [PubMed] [Google Scholar]
- 150.Xiao S., Brooks C.R., Sobel R.A., Kuchroo V.K. Tim-1 Is Essential for Induction and Maintenance of IL-10 in Regulatory B Cells and Their Regulation of Tissue Inflammation. J. Immunol. 2015;194:1602–1608. doi: 10.4049/jimmunol.1402632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ding Q., Yeung M., Camirand G., Zeng Q., Akiba H., Yagita H., Chalasani G., Sayegh M.H., Najafian N., Rothstein D.M. Regulatory B cells are identified by expression of TIM-1 and can be induced through TIM-1 ligation to promote tolerance in mice. J. Clin. Investig. 2011;121:3645–3656. doi: 10.1172/JCI46274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.DiLillo D.J., Matsushita T., Tedder T.F. B10 cells and regulatory B cells balance immune responses during inflammation, autoimmunity, and cancer. Ann. N. Y. Acad. Sci. 2010;1183:38–57. doi: 10.1111/j.1749-6632.2009.05137.x. [DOI] [PubMed] [Google Scholar]
- 153.Lee B.O., Haynes L., Eaton S.M., Swain S.L., Randall T.D. Faculty of 1000 evaluation for The biological outcome of CD40 signaling is dependent on the duration of CD40 ligand expression: Reciprocal regulation by interleukin (IL)-4 and IL-12. J. Exp. Med. 2002;196:693–704. doi: 10.1084/jem.20020845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Miyashita T., McIlraith M.J., Grammer A.C., Miura Y., Attrep J.F., Shimaoka Y., Lipsky P.E. Bidirectional regulation of human B cell responses by CD40-CD40 ligand interactions. J. Immunol. 1997;158:4620–4633. [PubMed] [Google Scholar]
- 155.Wang Y., Yu X., Lin J., Hu Y., Zhao Q., Kawai T., Taubman M.A., Han X. B10 Cells Alleviate Periodontal Bone Loss in Experimental Periodontitis. Infect. Immun. 2017;85:e00335-17. doi: 10.1128/IAI.00335-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Yu P., Hu Y., Liu Z., Kawai T., Taubman M.A., Li W., Han X. Local Induction of B Cell Interleukin-10 Competency Alleviates Inflammation and Bone Loss in Ligature-Induced Experimental Periodontitis in Mice. Infect. Immun. 2016;85:e00645-16. doi: 10.1128/IAI.00645-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Shi T., Jin Y., Miao Y., Wang Y., Zhou Y., Lin X. IL-10 secreting B cells regulate periodontal immune response during periodontitis. Odontology. 2019:1–8. doi: 10.1007/s10266-019-00470-2. [DOI] [PubMed] [Google Scholar]
- 158.Zhang J., Lapato A., Bodhankar S., Vandenbark A.A., Offner H. Treatment with IL-10 producing B cells in combination with E2 ameliorates EAE severity and decreases CNS inflammation in B cell-deficient mice. Metab. Brain Dis. 2015;30:1117–1127. doi: 10.1007/s11011-015-9661-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Mauri C., Menon M. Human regulatory B cells in health and disease: Therapeutic potential. J. Clin. Investig. 2017;127:772–779. doi: 10.1172/JCI85113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Mauri C., Menon M. The expanding family of regulatory B cells. Int. Immunol. 2015;27:479–486. doi: 10.1093/intimm/dxv038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hong M., Liao Y., Liang J., Chen X., Li S., Liu W., Gao C., Zhong Z., Kong D., Deng J., et al. Immunomodulation of human CD19+CD25high regulatory B cells via Th17/Foxp3 regulatory T cells and Th1/Th2 cytokines. Hum. Immunol. 2019;80:863–870. doi: 10.1016/j.humimm.2019.05.011. [DOI] [PubMed] [Google Scholar]
- 162.Mohib K., Cherukuri A., Zhou Y., Ding Q., Watkins S.C., Rothstein D.M. Antigen-dependent interactions between regulatory B cells and T cells at the T:B border inhibit subsequent T cell interactions with DCs. Am. J. Transplant. 2020;20:52–63. doi: 10.1111/ajt.15546. [DOI] [PMC free article] [PubMed] [Google Scholar]