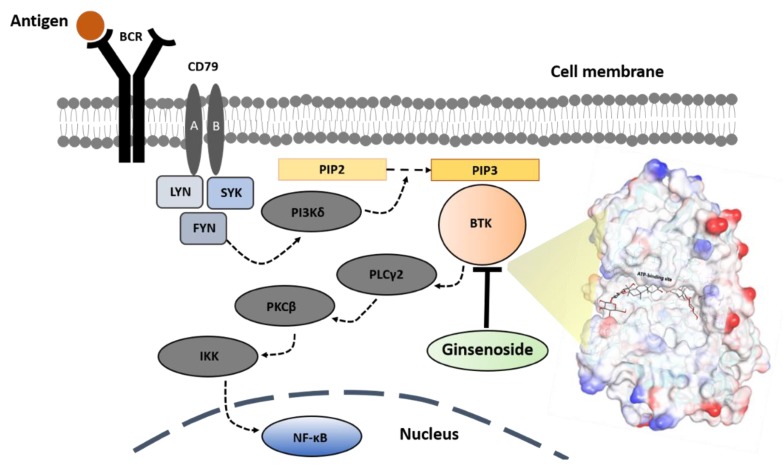
Figure 9.
A proposed mechanism of antigen-dependent B-cell receptor (BCR) signal transduction [16] and its targeting by a small molecule-based inhibitor (ginsenoside Rb3). Antigen binding to the BCR initiates kinase-mediated signal transduction and the aggregation of the BCR with its co-receptors (CD79A and CD79B), which become phosphorylated by the recruited tyrosine kinases, Lck/Yes novel tyrosine kinase (LYN) and spleen tyrosine kinase (SYK). SYK activates phosphoinositide 3-kinase (PI3Kδ), which converts phosphatidylinositol 4,5-bisphosphate (PIP2) to phosphatidylinositol 3,4,5-triphosphate PIP3. PIP3 serves as a docking site for the cytoplasmic kinase, Bruton’s tyrosine kinase (BTK). Thereby, BTK phosphorylates and activates phospholipase C gamma 2 (PLC2γ), which produces a set of second messengers to activate protein kinase C beta (PKCβ). PKCβ phosphorylates IκB kinase (IKK) to activate nuclear factor B (NF-κB) transcription factors that regulate the gene expression necessary for B cell survival and proliferation. Through the bioassay and molecular docking result, the Rb3 molecule was selected as a novel and highly potent inhibitor of the BTK enzyme.