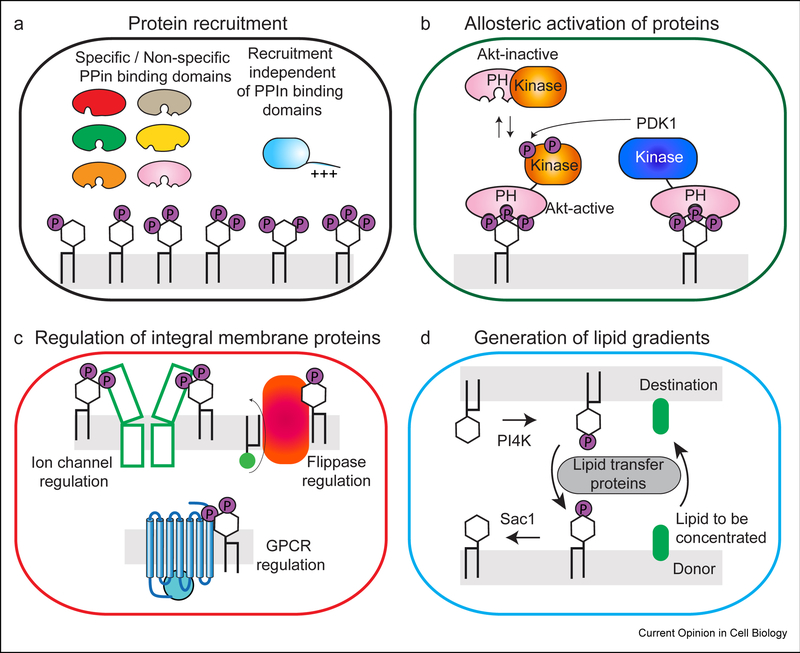
Figure 2: Phosphoinositides roles in protein recruitment/allosteric activation, modulation of integral membrane proteins, and lipid transport.
A. Role of Phosphoinositides in the recruitment of proteins to specific intracellular locations. Many PPIn binding domains have been identified, although many of these domains have varying levels of specificity, and also frequently require coincidence detection of other signals (including both additional lipid and protein binding partners). Phosphoinositides can also regulate protein recruitment outside of lipid binding domains, including polybasic stretches, and non-canonical lipid binding sites.
B. Roles of phosphoinositides in allosteric activation of signaling enzymes. Example of the allosteric activation of the pro-growth kinase Akt (PKB) downstream of PIP3, where PIP3 binding to the PH domain disrupts an inhibitory PH-kinase interface, followed by PIP3 activated phosphorylation of Akt by phosphoinositide dependent kinase 1 (PDK1).
C. Phosphoinositides are key regulators of integral membrane proteins, including ion channels, G-protein coupled receptors, and lipid scramblases, flippases and floppases. Phosphoinositides can regulate integral membrane function through allosteric conformational changes and/or through modulating their coupling to protein binding partners.
D. Phosphoinositides can mediate the transport of lipids against their concentration gradient through the coordinated action of lipid kinases, phosphatases, and lipid transport proteins (LTPs).