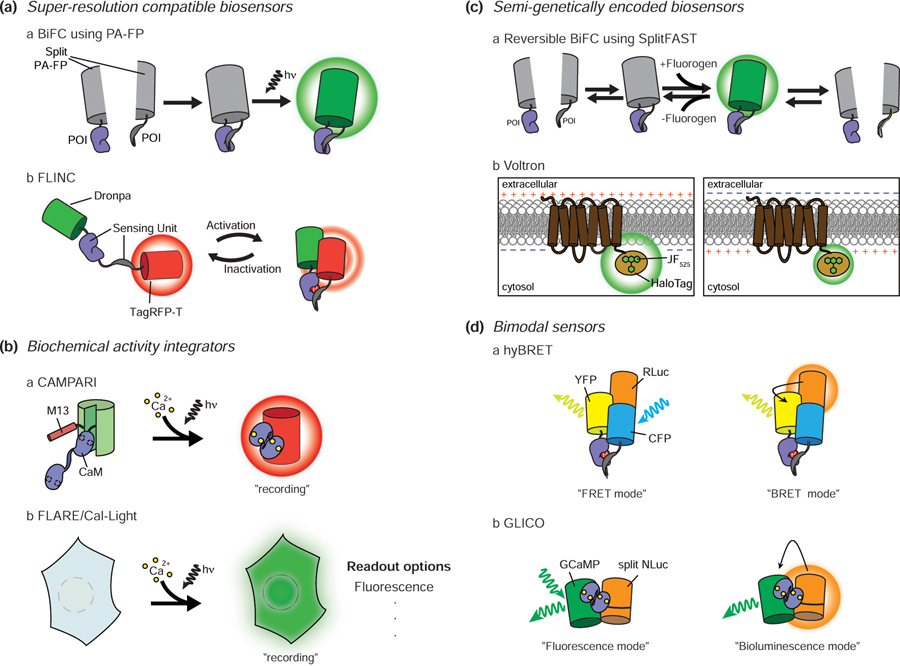
Figure 2.
Recent advances in biosensors. A. Super-resolution compatible biosensors. Bimolecular fluorescence complementation (BiFC) using photoactivatable FPs combined with STORM/PALM imaging enables super-resolution PPI observation (a). TagRFP-T fluorescence shows increasing fluctuations as the proximity between Dronpa and TagRFP-T is closed by the sensing unit. This allows the use of pcSOFI, a super-resolution technique, to observe signaling activity at subdiffraction spatial resolution (b). B. Biochemical activity integrators. CaMPARI is a GCaMP-based Ca2+ sensor that features a green-to-red photoconvertible FP. The sensor undergoes irreversible green-to-red photoconversion only when exposed to both violet light and Ca2+. Thus, the red color persists even when Ca2+ levels drop, allowing post hoc analysis of signaling (a). When FLARE/Cal-Light is exposed to Ca2+ and blue light, a transcription factor that turns on the reporter translocates into the nucleus. Unlike CaMPARI, this system can be used with a variety of readouts (b). C. Semi-genetically encoded biosensors. SplitFAST is suitable for showing dynamic PPIs because of its reversible interaction. Conventional BiFC cannot report PPI dynamics due to the irreversible nature of FP complementation, whereas splitFAST makes this possible (a). New synthetic dyes with improved brightness and stability help to overcome the limitations of the low delivery efficiency of synthetic dyes. Voltron is based on the voltage sensor Ace2N and new bright synthetic dyes such as JF525. This drastically improved in vivo voltage imaging in animals (b). D. Bimodal sensors. hyBRET sensors combine a FRET sensor with a bioluminescent protein (RLuc). In the absence of the luciferase substrate, the signaling activity can be measured in FRET mode, while addition of substrate allows monitoring in BRET mode (a). GLICO combines GCaMP with splitNLuc. In the presence of Ca2+, the GLICO sensing unit undergoes a conformational change that modulates cpGFP fluorescence and leads to splitNLuc complementation. Therefore, Ca2+ levels can be monitored via GFP emission caused by either external illumination (fluorescence mode) or luciferin addition to induce BRET between NLuc and cpGFP (bioluminescence mode) (b). PA: photoactivatable, hn: light, POI: protein of interest.