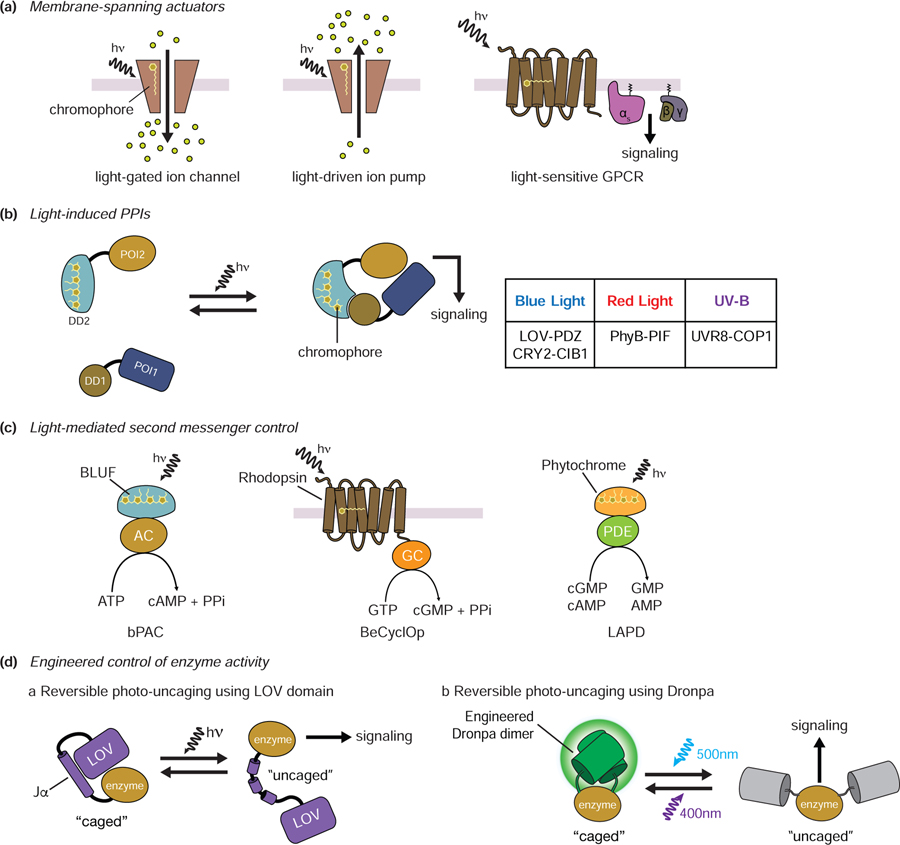
Figure 3.
Components and designs of optogenetic tools. A. Membrane-spanning actuators. Rhodopsin can act as a membrane-spanning actuator in the form of a light-driven ion channel, an ion pump, or a GPCR. The covalently bound chromophore of rhodopsin can trigger a conformational change in rhodopsin upon light illumination. B. Light-induced PPIs. Light-dependent modulation of protein translocation and/or signaling perturbation utilizes light-induced PPIs between photoreceptors and their effectors. Representative light-induced PPIs are shown in the table. C. Light-mediated second messenger control. In bPAC, adenylyl cyclase bound to the BLUF domain is activated by light to produce cAMP. A guanylyl cyclase-containing rhodopsin from Blastocladiella emersonii (BeCyclOp) similarly produces cGMP when activated by light. The red-light-activated phosphodiesterase (LAPD) degrades cGMP and cAMP. D. Engineered control of enzyme activity. The Jα helix of the AsLOV domain is unfolded by light, allowing enzyme “caging” and “uncaging” (a). Photodissociable dimeric Dronpa (pdDronpa1) can form dimers upon 500 nm light and dissociate upon 400nm light. This feature also allows reversible control of protein cagin/uncaging by light (b). hn: light, POI: protein of interest; DD: dimerization domain, AC: adenylyl cyclase, GC: guanylyl cyclase, PDE: phosphodiesterase