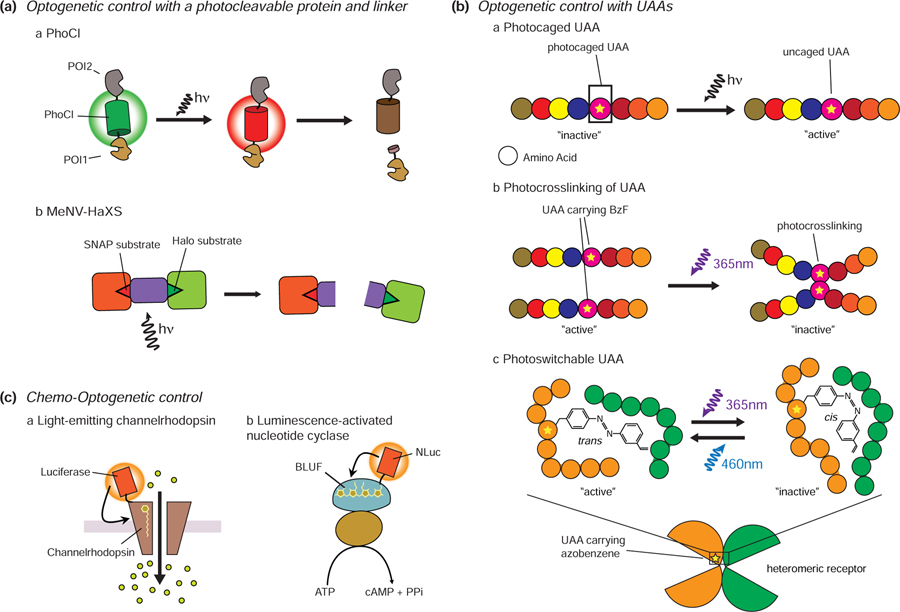
Figure 4.
Recent advances in optogenetic tools. A. Photocleavable protein and linker. The excitation of PhoCl by 400 nm light leads to its photoconversion and backbone cleavage. Proteins conjugated to PhoCl will thus translocate upon PhoCl cleavage (a). MeNV-HaXS is a photocleavable chemical dimerization system. HaloTagged and SNAP-tagged proteins that are tethered by this linker can be dissociated using 360 nm light irradiation (b). B. Optogenetic control with UAAs. Substitution of functionally critical amino acids with photocaged UAAs can be used to inhibit a target protein until light irradiation restores protein function (a). UAAs carrying BzF can induce photocrosslinking upon UV stimulation. The use of photocrosslinking UAAs can enable light-mediated inhibition of protein function (b). Azobenzene moieties can undergo reversible cis/trans photoisomerization in response to blue and UV light. Incorporating UAAs containing azobenzene moieties allows reversible cis/trans photoisomerization of an amino acid side chain, enabling reversible control of protein activity (c). C. Chemo-optogenetic control. Luminopsin combines channelrhodopsin with a bioluminescent protein. Bioluminescence from the luciferase can induce conformational changes in channelrhodopsin without the need for external illumination (a). Similarly, a luminescence-activated adenylyl cyclase also combines a bioluminescent protein (NLuc) with bPAC (b).