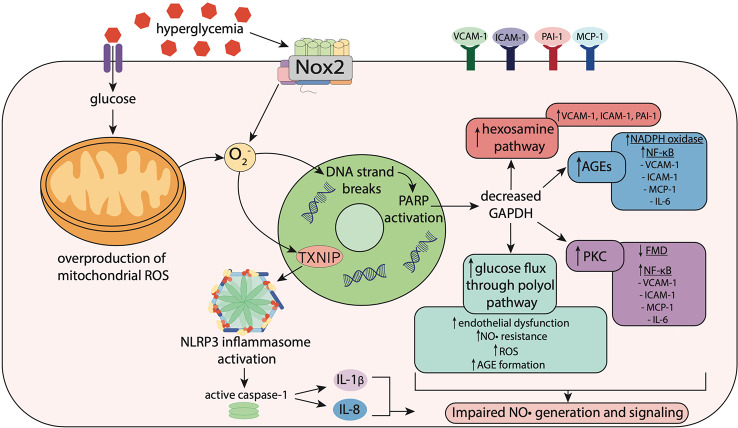
Figure 2.
Hyperglycemia-induced oxidative stress impairs nitric oxide signaling. Hyperglycemia induces oxidative stress via increased activity of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (Nox) enzymes including Nox2, resulting in superoxide (O2 −) generation. In mitochondria, hyperglycemia increases reactive oxygen species (ROS) production including O2 −, which stimulates thioredoxin-interacting protein (TXNIP) expression. TXNIP promotes activation of the NLRP3 inflammasome, which activates caspase-1, resulting in the maturation and secretion of the pro-inflammatory cytokines, interleukin-1β (IL-1β) and interleukin-18 (IL-18). O2 − also causes strand breaks in DNA, leading to activation of poly (ADP-ribose) polymerase (PARP), which reduces activity of glyceraldehyde-3 phosphate dehydrogenase (GAPDH). Decreased GAPDH activity leads to overactivation of the hexosamine pathway, upregulation of protein kinase C (PKC), elevated glucose flux through the polyol pathway and increased formation of advanced glycation end-products (AGEs). This leads to activation of Nox and NF-κB signaling, resulting in increased expression of pro-inflammatory and pro-atherogenic mediators including vascular adhesion molecule-1 (VCAM-1), intracellular adhesion molecule-1 (ICAM-1), monocyte chemoattractant protein-1 (MCP-1), plasminogen activator inhibitor-1 (PAI-1) and interleukin-6 (IL-6). Upregulation of these pathways results in impaired flow-mediated vasodilation, reflecting both endothelial dysfunction and nitric oxide (NO•) resistance.