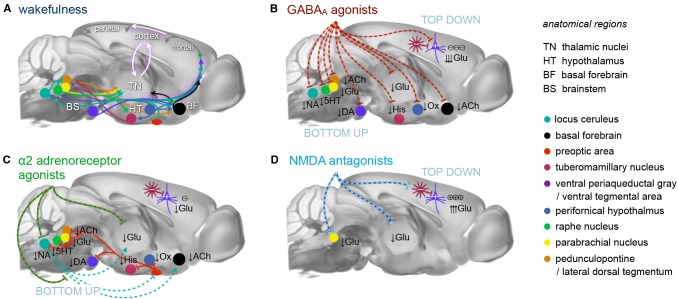
Figure 1.
Principal mechanisms of anesthesia. (A) Brain states are governed by a highly interconnected assembly of subcortical (arousal) nuclei. These nuclei employ distinct transmitter systems, including glutamate (Glu), noradrenaline (NA), serotonin (5HT), dopamine (DA), acetylcholine (ACh), histamine (His), and orexin (Ox). They project to the cortex directly and via higher-order nuclei of the thalamus. Thalamus and cortex are densely interconnected and heavily exchange information. Synchronous rhythmic thalamocortical activity can set the phase relations of distant cortical areas. Similar phase relations facilitate information transfer across the cortex and from frontal to parietal regions. All anesthetics generate distinct thalamocortical rhythms and alter the phase relationship of transmitting and receiving areas, leading to a successive breakdown of cortico-(thalamo-)cortical communication and eventually loss of consciousness. (B) GABAA agonists, including volatile ethers, affect brain states both top-down and bottom-up by inhibiting excitatory neurons in subcortical nuclei and directly in the cortex. (C) α2AR agonists exert their effects bottom-up mainly by inhibiting the locus coeruleus, which leads to a disinhibition of sleep promoting neurons in the preoptic area of the hypothalamus. (D) N-methyl-d-aspartate (NMDA) antagonists act primarily top-down in a dual mode: at low doses by inhibiting inhibitory interneurons leading to cortical excitation, and at higher dosages by also inhibiting excitatory cortical pyramidal neurons. Further suppression of nociception and arousal is mediated by blocking the parabrachial nucleus. These three routes present the major principles of anesthesia. All anesthetics in current use in preclinical fMRI act in one of these ways. Inspired by Franks (2008), Lee and Dan (2012) and Akeju and Brown (2017), plotted on an MR reference template of the Allen mouse brain atlas (Bakker et al., 2015).