Figure 3.
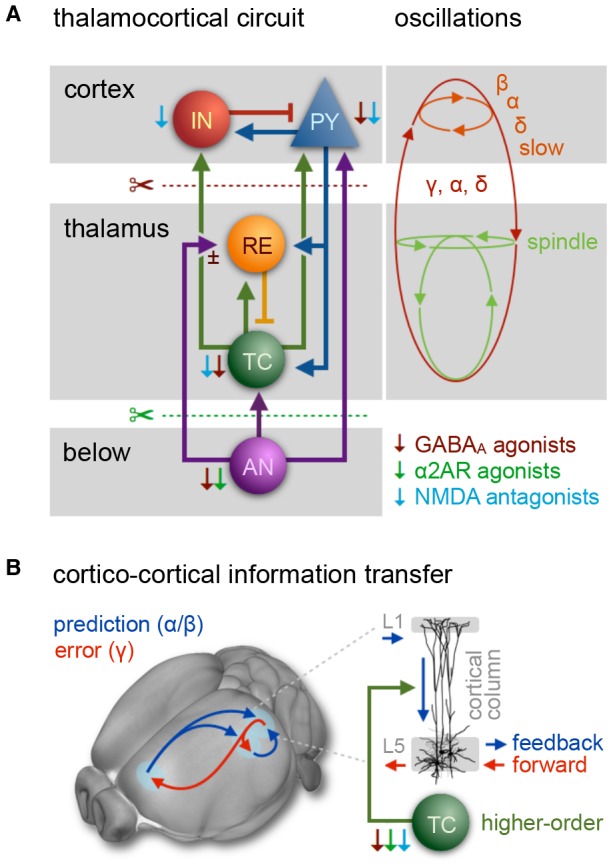
The thalamocortical circuit as wave generating unit, and cortical information transfer. (A) Scheme of thalamocortical circuit displays major interactions between GABAergic inhibitory interneurons (IN) and excitatory pyramidal cells (PY) in the cortex and thalamic GABAergic inhibitory reticular cells (RE) and excitatory thalamocortical neurons (TC). Arousal nuclei (AN) drive cortical desynchronization via cortical and thalamocortical circuits, including biphasic excitatory–inhibitory modulation (Sun et al., 2013). Anesthetic classes reduce the membrane potential of their respective target neurons (indicated by thin color-coded arrows), leading to inhibition and rebound spiking. Cortical oscillations are produced by synchronized periodic activity between two or more cell types. Transection below cortex (red scissors) leads to slow wave generation in the cortex; transection below thalamus (cerveau isolé, green scissors) leads to generation of sleep-like spindles and slow-delta waves. Anesthesia-induced oscillations: slow (<1 Hz), δ (1–4 Hz), α (8–15 Hz), β (15–30 Hz), γ (25–35 Hz), spindle (9–16 Hz). (B) Fronto-parietal information transfer along the cortical hierarchy. Feedback information (blue) is conveyed from higher-order frontal areas to primary sensory areas in the α/β band, feedforward information flow (red) from primary to higher-order areas in the γ band. In the predictive coding scheme feedback signals are considered predictive models of upcoming sensory states, whereas feedforward information represents the error resulting from a mismatch of a predictive model with an actual state. Feedback transmitted via long-range projections enters cortical columns at the dendritic trees of pyramidal cells in layer 1 (L1). The signal is transmitted to the soma of the same cells in layer 5, which requires driving input of higher-order TC nuclei. Feedback information transfer breaks down with the inhibition of higher-order TC nuclei at increasing depth of anesthesia. (A) Inspired by Ching and Brown (2014) and others. (B) Left panel: adapted from Sikkens et al. (2019). Note that visual higher-order areas in mice differ from those of primates, as reviewed in Glickfeld and Olsen (2017); Pennartz et al. (2019). Right panel: based on Suzuki and Larkum (2020).
