Figure 2.
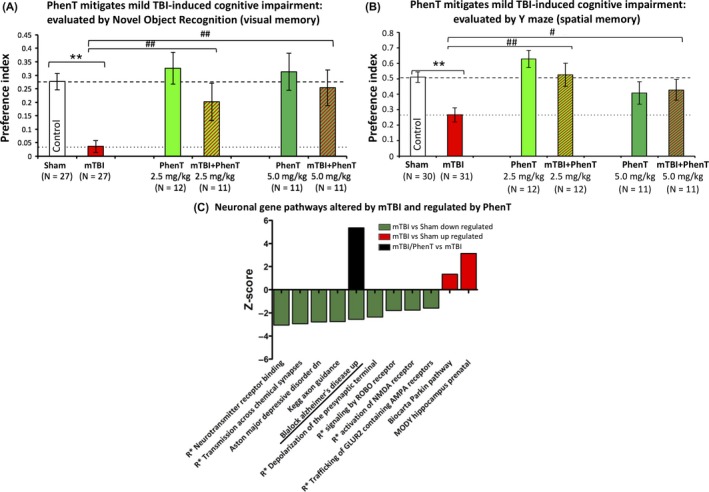
mTBI‐induces memory deficits and triggers gene pathways leading to AD that PhenT reverses. A, mTBI‐induced an impairment in visual memory (NOR) vs control uninjured (Sham) mice (**P < .01) when evaluated 7 days postinjury. PhenT significantly ameliorated this damage (both doses ##P < .01) when administered twice daily postinjury for 5 days and washed out for 2 days prior to cognitive evaluations. Data are mean ± SEM; one‐way ANOVA revealed a significant effect between groups (F(5,98) = 7.770, P = .000). Fisher's LSD post hoc analysis revealed that the preference index of the “mTBI” group was significantly lower than all others (##P < .01).25 B, mTBI‐induced deficits in spatial memory compared with control uninjured (Sham) animals (**P < .01), as evaluated by the Y‐maze paradigm. PhenT significantly mitigated this (## P < .01 for 2.5 mg/kg and # P < .05 for 5 mg/kg). One‐way ANOVA revealed a significant effect between groups [F(5,105) = 6.190, P = .000]. Fisher's LSD post hoc analysis revealed that the preference index of the “mTBI” group was significantly lower than all other groups other than PhenT 5 mg/kg (*P < .05, **P < .01). C, CNS neuronal canonical and noncanonical pathways observed to be triggered by mTBI when compared to sham animals, and the effects of PhenT postinjury treatment (R* refers to Reactome). The majority of pathways were observed to be downregulated by mTBI, with only two that were upregulated. Two pathways were associated with neurodegenerative disease: “Blalock Alzheimer's Disease Up” and “Biocarta Parkin Pathway”. Whereas postinjury treatment of animals with PhenT had no substantial effect on the majority of pathways; notably, the AD‐related pathway was regulated by this drug. Specifically, PhenT posttreatment reversed the effects triggered by mTBI on the AD pathway25
