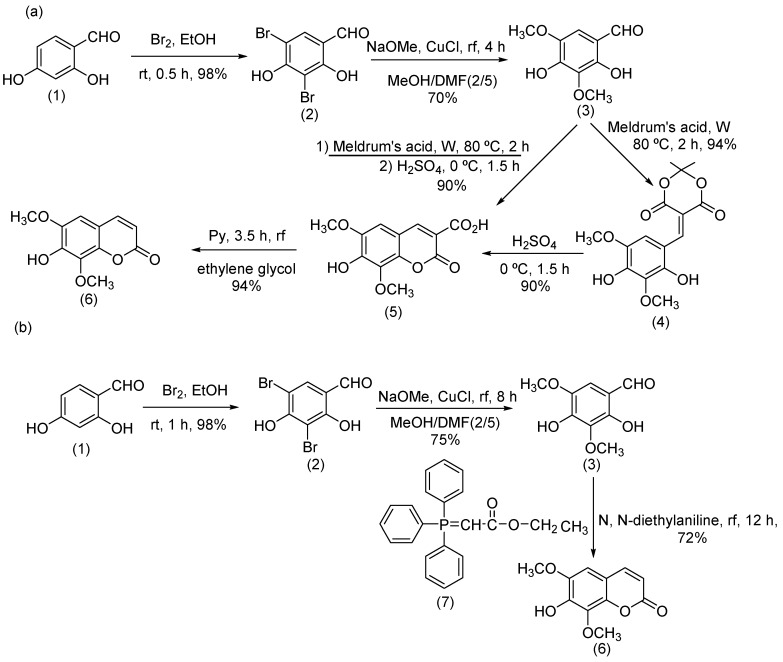
Scheme 1.
The Synthesis procedure of IF in the modified Knoevenagel (a) and Wittig (b) methods. (1): 2,4-Dihydroxybenzaldehyde, (2): 3,5-Dibromo-2,4-dihydroxybenzaldehyde, (3): 2,4-Dihydroxy-3,5-dimethoxybenzaldehyde, (4): 5-(2,4-Dihydroxy-3,5-dimethoxybenzylidene)- 2,2-dimethyl-1,3-dioxane-4,6-dione, (5): 7-Hydroxy-6,8-dimethoxy-2-oxo-2H- chromene-3-carboxylicacid, (6): 7-Hydroxy-6,8-dimethoxy-2H-chromen-2-one (Isofraxidin), (7): ethyl (triphenylphosporanylidene) acetate, EtOH: Ethanol, rt: room temperature, MeOH: Methanol, NaOMe: Sodium methoxide, CuCl: Copper chloride, DMF: Dimethylformamide, rf: reflux, W: water, H2SO4: Sulfuric acid, h: hour, Py: pyridine.