Table 1.
Results of syntheses of monosubstituted piperazine derivatives using different synthetic techniques.
| Reactant (R) | Molar Ratio 1 | Catal. | Proc. 2 | Time (hr) | Product | Aver. Yield 3 (%) |
|---|---|---|---|---|---|---|
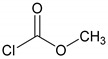
|
-: 1.3 | Cu(I) | A | 24 |
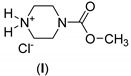
|
70 |
| - | - | B 4 | - | - | ||
| -: 1.1 | Ce(III) | C 4 | 0.58 | 61 | ||

|
1: 1.1 | - | A | 19 |

|
57 |
| 1: 1.2 | Ce(III) | A | 8 | 61 | ||
| 1: 1.2 | Cu(II) | B | 6 | 54 | ||
| 1: 1.2 | Cu(II) | C | 2.17 | 64 | ||

|
1: 2.2 | - | A | 16 |

|
62 |
| 1: 2.6 | Ce(III) | A | 7 | 60 | ||
| 1: 2 | B | 4 | 50 | |||
| 1.2: 2.7 | C | 0.17 | 61 | |||

|
0.2: 1.2 | Cu(II) | A | 8 |

|
71 |
| 0.2: 1.1 | B | 1.83 | 72 | |||
| - | C | - | - | |||

|
1: 1.1 | Cu(II) | A | 13 |

|
84 |
| 0.5: 1.1 | B | 2.5 | 67 | |||
| - | C | - | - | |||

|
0.5: 1.1 | Cu(II) | A | 14 |

|
88 |
| 0.5: 1.1 | B | 1 | 69 | |||
| - | C | - | - |
1 Molar ratios are listed as piperazine. 2HCl: reactant and are always related to 1 mol of anhydrous piperazine. 2 Proc. A follows literature method [46,47,48,49,50], proc. B (batch) and C (flow) proceed under MW irradiation. 3 Average yield of a recrystallized product with respect to anhydrous piperazine excepting reactions of methyl acrylate which is related to piperazine monohydrochloride. 4 The reaction proceeds at room temperature and thus only a simple flow mode using catalyst was applied.
