Abstract
Objective
To determine the frequency that non-first-line antibiotics, safety-net antibiotic prescriptions (SNAPS), and longer than recommended durations of antibiotics were prescribed for children ≥2 years of age with acute otitis media and examine patient and system level factors that contributed to these outcomes.
Study design
Children age ≥2 years with acute otitis media seen at Denver Health Medical Center outpatient locations from January to December 2018 were included. The percentages of patients who received first-line antibiotics, SNAPs, and recommended durations of antibiotics were determined. Factors associated with non-first-line and longer than recommended antibiotic durations were evaluated using multivariate logistic regression modeling.
Results
Of the 1025 visits evaluated, 98.0% were prescribed an antibiotic; only 4.5% of antibiotics were SNAPs. Non-first-line antibiotics were prescribed to 18.8% of patients. Most antibiotic durations (94.1%) were longer than the institution recommended 5 days and 54.3% were ≥10 days. Private insurance was associated with non-first-line antibiotics (aOR, 1.89; 95% CI, 1; 14-3.14, P = .01). Patients who were younger (2-5 years; aOR2.01; 95% CI, 1.32-3.05; P < .001) or seen in emergency/urgent care sites (aOR, 1.73; 95% CI, 1.26-2.38; P < .001) were more likely to receive ≥10 days of antibiotic compared with those in pediatric clinics.
Conclusions
Antibiotic stewardship interventions that emphasize the duration of antibiotic therapy as well as the use of SNAPs or observation may be higher yield than those focusing on first-line therapy alone. Numerous system and patient level factors are associated with off-guideline prescribing.
Antibiotics are the most commonly prescribed medications to children.1 Unfortunately, the overuse of antibiotics and the use of overly broad antibiotics have led to an alarming increase in antimicrobial-resistant organisms.2 Additionally, overuse of antibiotics and the use of overly broad antibiotics results in increased adverse drugs events,3 dysbiosis of the microbiome that places children at risk for future chronic diseases such as juvenile idiopathic arthritis,4 and inflammatory bowel disease,5 higher risk for community-acquired Clostridium difficile infections,6 and increased costs.7
Acute otitis media (AOM) is the most commonly cited indication for antibiotics in children accounting for 24% of all pediatric antibiotic prescriptions and 8.7 million prescriptions annually.1 By 3 years of age, 60% of children have had ≥1 episode of AOM and 24% have had ≥3 episodes.8 Thus, understanding prescribing habits for AOM is critical to designing effective strategies to improve overall pediatric antibiotic use.
For most children with AOM, antibiotics are of limited benefit.9 American Academy of Pediatrics 2013 guidelines recommend using safety-net antibiotic prescriptions (SNAP; also delayed antibiotic prescriptions) for children >6 months of age with mild to moderate unilateral AOM. SNAPs have been shown to reduce antibiotic use while maintaining patient satisfaction.10 Amoxicillin is recommended as first-line therapy for most children with AOM with a duration of 10 days for patients <2 years of age, 7 days for patients 2-5 years of age with mild to moderate infection, and 5-7 days for patients >5 years of age with mild to moderate infection.11 A duration of 10 days is recommended for children with severe infection (fever ≥102.2°F, moderate to severe otalgia, or otalgia ≥48 hours). In contrast, the 2018 National Institute of Health and Care Excellence (UK) guidelines focus on symptomatic care and recommend no antibiotic or a SNAP for the majority of children. If an antibiotic is prescribed, amoxicillin with duration of 5-7 days is recommended.12
For many childhood infections, including community-acquired pneumonia, shorter courses of antibiotics have been shown to be equally effective as longer courses.13,14 Similarly, studies suggest that for most children with AOM treatment failure at >30 days does not differ between those who receive 5 days of therapy and those who receive ≥7 days of therapy.15 However, though prior studies have evaluated the rates of broad-spectrum antibiotic use for AOM, the prescribing rates of SNAPs and duration of antibiotics prescribed for AOM remain largely unknown.16–18 Prior pediatric antimicrobial stewardship program interventions for AOM have focused predominately on reducing broad-spectrum antibiotic use.19 A complementary strategy to decrease antibiotic exposure is to use the shortest effective duration of antibiotics.20 Thus, we aimed to determine the frequency that non-first-line antibiotics, SNAP prescriptions, and longer than recommended durations of antibiotics were prescribed for children ≥2 years of age with AOM and examined patient and system level factors that contributed to these outcomes.
Methods
The study was completed at Denver Health (DH) Medical Center in Denver, Colorado. DH is a university-affiliated, community-based health care system that includes 27 federally qualified health care system clinics and serves as the safety-net health care system for the region. In 2018, racial and ethnic demographics for all patients who presented to DH were 58% white, 26% black, and 15% other/unknown race; 47% of patients identified as Hispanic.21 Among all DH patients, 16.0% were uninsured and 60.9% had Medicaid or Children’s Health Insurance Program.22
Children are seen in a variety of outpatient settings at DH, including a pediatric emergency department, 2 urgent care centers, 10 community-based health centers, and 17 school-based clinics. A formal antibiotic stewardship program has been in place since July 2008.23 Institution-specific clinical care guidelines for AOM that recommend the use of SNAPs and a duration of antibiotics of 7 days for children 2-5 years of age and 5 days for children ≥6 years of age with nonrecurrent infection without tympanic membrane perforation have been available online and in a mobile application since 2016.24 Current institutional guidelines recommend 5 days of therapy for patients ≥2 years of age.
Patient Population
Patients age 2-18 years of age seen at DH clinics, emergency department, or urgent care centers between January 1, 2018, and December 31, 2018, with an International Classification of Diseases, 10th edition, code for AOM were included (Table I; available at www.jpeds.com). Patients with a competing bacterial diagnosis that may warrant an antibiotic, such as a urinary tract infection or pneumonia, were excluded from the initial data pull.16 Patients were also excluded if they had received an antibiotic within 30 days before the visit or had a history of tympanostomy tubes because these patients were more likely to have recurrent or chronic infections. Patients who were not prescribed any antibiotic were excluded as first-line therapy and antibiotic duration could not be assessed for these patients; patients who were prescribed SNAPs were included. Patients who received intramuscular antibiotics or azithromycin were included in the analysis of first-line antibiotic prescriptions, but excluded from the analysis of duration of antibiotic therapy.
Table I.
International Classification of Disease, 10th edition, codes for AOM
| H65.00-.07 |
| H65.191-.199 |
| H66.001-.009 |
| H66.40-.43 |
| H66.90-.93 |
| H67.1-.3 |
| H67.9 |
Data Abstraction
Data were electronically and manually abstracted from the electronic health record (EHR; Epic, Verona, Wisconsin). Electronically abstracted data included demographics, history of sick visits and hospitalizations, diagnoses, antibiotics prescribed including dosage and duration of therapy, visit location, and allergies. Manually abstracted data included smoke exposure; history of tympanostomy tubes; SNAP data; patient signs and symptoms including conjunctivitis, fever, and infection laterality; trial of over-the-counter medications; and pneumococcal conjugate vaccine immunization status. Patients with tympanostomy tubes identified on manual abstraction were excluded. Insufficient data were available to abstract otalgia severity or tympanic membrane findings, including perforation.
Statistical Analyses
First, descriptive statistics demonstrating the percentage of non-first-line, longer than recommended, and SNAPs were calculated along with 95% Wald CIs. The total number of days of antibiotic exposure was calculated. Univariate and multivariate statistical analyses were completed to determine patient and system level risk factors for non-first-line antibiotic prescriptions; and longer than recommended antibiotic prescriptions. Amoxicillin was considered first-line therapy; all other agents were classified as non-first-line therapy. Because patients with recurrent or recently treated AOM were excluded from the study and the prevalence of penicillin allergy and conjunctivitis were low alternative antibiotics were not considered first-line, even though they may have been appropriate for a small subset of patients. For comparative analyses, recommended duration of therapy was defined as ≤7 days and longer than recommended duration of therapy was defined as ≥10 days.11
Patient- and system-level factors were compared across groups using χ2 and Fisher exact tests. Multivariable logistic regression models were developed using stepwise selection for variables with P < .01 on univariate analyses. Race and ethnicity were included in regression models, regardless of significance, because they have previously been shown to be associated with differences in AOM treatment.25,26 Best subset regression was used to verify the validity of the models. Results were summarized using aORs and Wald 95% CIs. Significance was defined as a P value of <.05. Statistical analysis was completed using SAS 9.4 (SAS Institute, Cary, North Carolina).
The project was completed as a baseline assessment for an antimicrobial stewardship quality improvement intervention and was reviewed by the Quality Improvement Committee of DH, which is authorized by the Colorado Multiple Institutional Review Board at the University of Colorado, Denver, and was determined not to be human subjects research.
Results
Between January and December of 2018, there were 1025 visits for children ages 2-18 years for AOM (Figure). After removing the visits that did not meet inclusion criteria, 926 were included in the analysis of first-line vs non-first-line antibiotic prescribing. After further excluding children who received azithromycin or an intramuscular antibiotic, the duration of therapy analysis included 911 visits. Overall, 98% of children (1005 of 1025) who presented with AOM were prescribed an antibiotic. Of those who met inclusion criteria, only 42 prescriptions (4.5%) were SNAPs.
Figure.
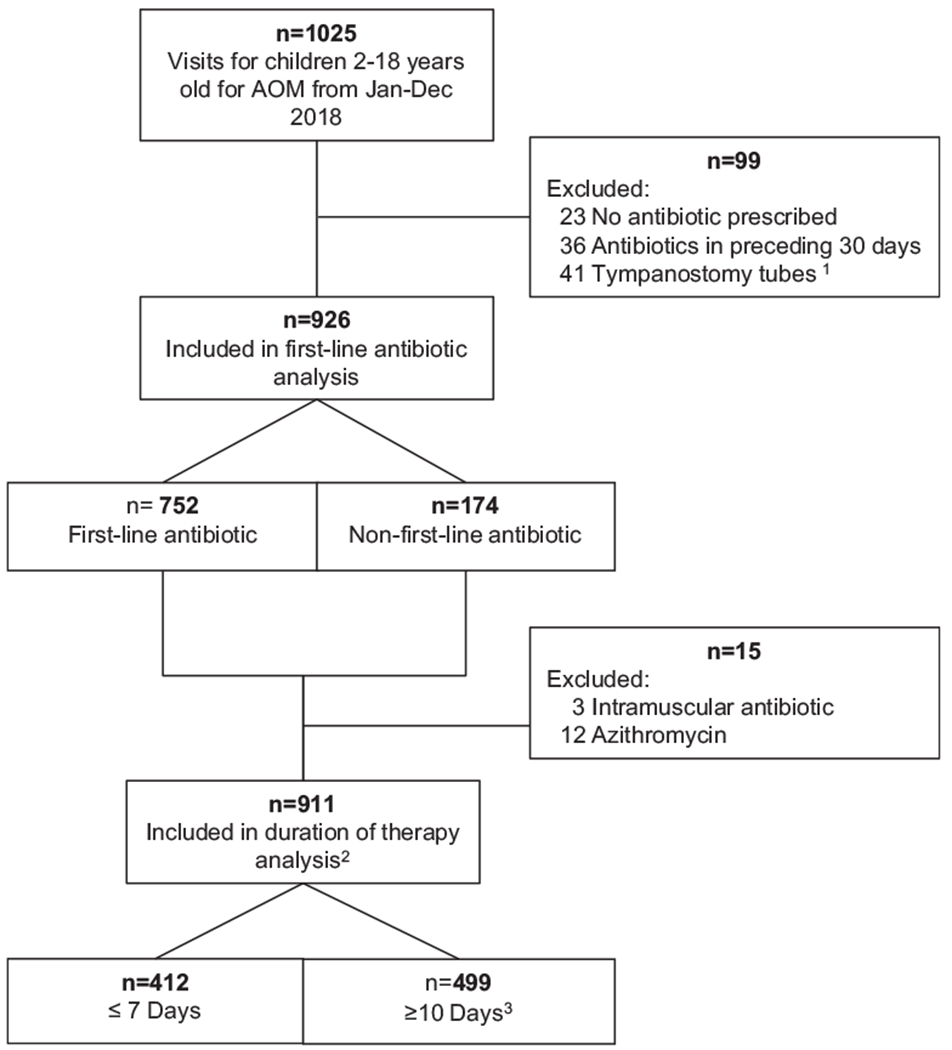
Visits and antibiotic prescribing for children 2-18 years old with AOM at DH Medical Center; January to December 2018. 1One patient had both tympanostomy tubes and received antibiotics in the prior 30 days. 2Includes patients who received safety net antibiotic prescriptions. 3No patients were prescribed 8 or 9 days of antibiotics.
Antibiotic Selection
Of 926 antibiotic prescriptions, 752 (81.2%) were for amoxicillin, the first-line antibiotic (Table II). Amoxicillin-clavulanate was the most common non-first-line antibiotic prescribed (174, or 18.8% of non-first-line antibiotics; Table II). Of patients prescribed amoxicillin-clavulanate, 40 (23.0%) had conjunctivitis noted during their visit.
Table II.
Types of antibiotics prescribed and duration of therapy for children 2-18 years old who received antibiotics for AOM at DH Medical Center, January-December 2018
| Antibiotics prescribed | Total (n = 926) | 2-5 years (n = 537) | 6-10 years (n = 258) | >10 years (n = 131) |
|---|---|---|---|---|
| First line (amoxicillin) | 752 (81.2) | 443 (82.5) | 214 (82.9) | 95 (72.5) |
| Non-first line | 174 (18.8) | 94 (17.5) | 44 (17.1) | 36 (27.5) |
| Amoxicillin-clavulanate | 101 (10.9) | 60 (11.2) | 21 (8.1) | 20 (15.3) |
| Cefdinir | 51 (5.5) | 26 (4.8) | 17 (6.6) | 8 (6.1) |
| Azithromycin | 12 (1.3) | 4 (0.7) | 4 (1.6) | 4 (3.1) |
| Other* | 10 (1.1) | 4 (0.7) | 2 (0.8) | 4 (3.1) |
| Days of therapy | (n = 911) | (n = 532) | (n = 254) | (n = 125) |
| 5 | 54 (5.9) | 13 (2.4) | 24 (9.4) | 17 (13.6) |
| 7 | 362 (39.7) | 196 (36.8) | 107 (42.2) | 59 (47.2) |
| 10 | 493 (54.1) | 322 (60.5) | 123 (48.4) | 48 (38.4) |
| 14 | 2 (0.2) | 1 (0.3) | 0 (0.0) | 1 (0.8) |
Values are number (%).
Includes cephalexin (n = 1), ceftriaxone (n = 3), cefuroxime (n = 1), and clindamycin (n = 5).
Bilateral infections, private insurance, >3 sick visits in the prior year, and penicillin allergy were independently associated with non-first-line prescribing in the multivariate logistic regression model (Table III). Patients who were age 2-5 years and female were more likely to receive first-line antibiotics. Antibiotic choice did not vary by patient race or ethnicity.
Table III.
Characteristics of children 2-18 years old with AOM who received first-line antibiotics compared with those who received non-first-line antibiotics at DH Medical Center, January to December 2018
| Characteristics | First-line antibiotics (n = 752) | Non-first-line antibiotics (n = 174) | P value* |
|---|---|---|---|
| Age (years) | .03 | ||
| 2-5 | 443 (58.9) | 94 (54.0) | |
| 6-10 | 214 (28.5) | 44 (25.3) | |
| >10 | 95 (12.6) | 36 (20.7) | |
| Female | 376 (50.0) | 74 (42.5) | .08 |
| Non-white/other race | 245 (32.6) | 53 (30.5) | .59 |
| Hispanic | 549 (73.0) | 127 (73.0) | .99 |
| Private insurance | 66 (8.8) | 27 (15.5) | .008 |
| Location | .93 | ||
| Pediatric clinic | 345 (45.9) | 79 (45.4) | |
| Nonpediatric clinic | 158 (21.0) | 35 (20.1) | |
| Emergency/urgent care | 249 (33.1) | 60 (34.5) | |
| SNAPs | 35 (4.7) | 7 (4.0) | .72 |
| Penicillin allergy | 4 (0.5) | 7 (4.0) | .001 |
| Received ≥1 dose PCV13 | 603 (80.2) | 126 (72.4) | .02 |
| Received ≥2 doses any PCV | 682 (73.7) | 151 (86.8) | .12 |
| Smoke exposure | 103 (13.7) | 29 (16.7) | .31 |
| Hospitalized in prior year | 23 (3.1) | 2 (1.2) | .20 |
| ≥3 Sick visits in prior year | 277 (36.8) | 69 (39.7) | .49 |
| Bilateral infection | 146 (19.4) | 47 (27.0) | .03 |
| Failed OTC medication trial | 52 (6.9) | 11 (6.3) | .78 |
| Fever† | 348 (46.3) | 73 (42.0) | .30 |
|
Multivariate logistic model for first-line vs non-first-line antibiotics‡ | |||
| Final model | |||
| Age, years | |||
| 2-5 | 0.55 (0.29-1.05) | .05 | |
| 6-10 | 0.52 (0.27-0.99) | .07 | |
| >10 | Referent | ||
| Female | 0.70 (0.50-0.99) | .04 | |
| Non-white/other race | 0.94 (0.64-1.38) | .77 | |
| Hispanic | 1.03 (0.68-1.54) | .90 | |
| Private insurance | 1.89 (1.14-3.14) | .01 | |
| Penicillin allergy | 8.13 (2.3-28.7) | .001 | |
| Received ≥1 dose PCV13 | 1.09 (0.63-1.89) | .76 | |
| Bilateral infection | 1.56 (1.06-2.31) | .02 | |
OTC, over-the-counter; PCV, pneumococcal conjugate vaccine.
Values that are statistically significant are in bold.
Values are number (%) or aOR (Wald 95% CI).
Calculated using 2-sided χ2 or Fisher exact test with significance defined as P < .05.
Fever subjectively reported by parents or documented as ≥100.4°F.
Factors with P < .1 on univariate analysis were included in the logistic regression model along with race and ethnicity regardless of significance.
Duration of Therapy
Of the 911 prescriptions included in the duration of therapy analysis, only 54 (5.9%) received a duration of 5 days of antibiotic therapy (Table III). In contrast, 362 (39.7%) received 7 days, 493 (54.1%) received 10 days, and 2 received 14 days of antibiotics. No patients received 8 or 9 days of antibiotic therapy. In total 7772 antibiotic exposure days were prescribed. Younger age (2-5 years), female sex, bilateral infections, and evaluation at an emergency department or urgent care were independent predictors of longer than recommended antibiotic durations in the multivariate analysis (Table IV). Fever was not significantly associated with longer than recommended antibiotic durations (P = .45). Patients who received SNAP prescriptions had failed a trial of over-the-counter analgesics, or were Hispanic and were less likely to be prescribed longer than recommended antibiotic courses.
Table IV.
Characteristics of children 2-18 years old with AOM who received ≤7 days of antibiotics compared with those who received ≥10 days at DH Medical Center, January-December 2018
| Characteristics | ≤7 Days of therapy (n = 412) | ≥10 Days of therapy (n = 499) | P value* |
|---|---|---|---|
| Age, years | <.0001 | ||
| 2-5 | 208 (50.5) | 324 (64.9) | |
| 6-10 | 130 (31.6) | 124 (24.9) | |
| >10 | 74 (18.0) | 51 (10.2) | |
| Female | 187 (45.4) | 256 (51.3) | .08 |
| Non-white/other race | 129 (31.3) | 164 (32.9) | .62 |
| Hispanic | 310 (75.2) | 351 (70.3) | .10 |
| Private insurance | 35 (8.5) | 56 (11.2) | .17 |
| Location | <.01 | ||
| Pediatric clinic | 212 (51.5) | 205 (41.1) | |
| Nonpediatric clinic | 87 (21.1) | 104 (20.8) | |
| Emergency/urgent care | 113 (27.4) | 190 (38.1) | |
| First-line antibiotic | 352 (85.4) | 400 (80.2) | .04 |
| SNAPs | 26 (6.3) | 14 (2.8) | .01 |
| Penicillin allergy | 3 (0.7) | 8 (1.6) | .36 |
| Received ≥1 dose PCV13 | 317 (76.9) | 403 (80.8) | .16 |
| Received ≥2 doses any PCV | 369 (89.6) | 450 (90.2) | .76 |
| Smoke exposure | 54 (13.1) | 75 (15.0) | .41 |
| Hospitalized in prior year | 10 (2.4) | 15 (3.0) | .59 |
| ≥3 Sick visits in prior year | 280 (68.0) | 293 (58.7) | .02 |
| Bilateral infection | 69 (16.8) | 122 (24.5) | <.01 |
| Failed OTC medication trial | 37 (9.0) | 23 (4.6) | <.01 |
| Fever† | 182 (44.2) | 233 (46.7) | .45 |
| Multivariate logistic model for ≤7 vs ≥10 days of therapy‡ | |||
| Final model | |||
| Age, years | |||
| 2-5 | 2.01 (1.32-3.05) | <.001 | |
| 6-10 | 1.28 (0.82-2.02) | .28 | |
| >10 | Referent | ||
| Female | 1.32 (1.01-1.74) | .04 | |
| Non-white/other race | 1.16 (0.86-1.58) | .34 | |
| Hispanic | 0.72 (0.52-1.00) | .05 | |
| Location | |||
| Emergency/urgent Care | 1.73 (1.26-2.38) | <.001 | |
| Nonpediatric clinic | 1.16 (0.81-1.67) | .42 | |
| Pediatric clinic | Referent | ||
| >3 Sick visits in prior year | 1.35 (1.01-1.80) | .04 | |
| Non-first-line antibiotic | 1.44 (1.00-2.08) | .05 | |
| Safety net antibiotic | 0.48 (0.24-0.96) | .04 | |
| Failed OTC medication | 0.56 (0.32-0.99) | .05 | |
| Bilateral infection | 1.69 (1.20-2.38) | <.01 | |
Values that are statistically significant are in bold.
Values are number (%) or aOR (Wald 95% CI).
Calculated using 2-sided χ2 or Fisher exact test with significance defined as P < .05.
Fever subjectively reported by parents or documented as ≥100.4°F.
Factors with P < .1 on univariate analysis were included in the logistic regression model along with race and ethnicity regardless of significance.
Discussion
In this study of >900 children age ≥2 years presenting with AOM, 98% were prescribed an antibiotic. Nearly 20% of the prescriptions were for non-first-line antibiotics. Most prescriptions (94%) were for longer than the current institution recommended duration of therapy and more than one-half (54%) were for ≥10 days of therapy. By logistic regression, numerous factors including age, sex, and laterality were independently associated with non-first-line antibiotic selection and longer than recommended durations of therapy.
To date, most institutional and national efforts to improve antibiotic prescribing for AOM have focused on the use of first-line rather than broader spectrum antibiotics.19 In our system, most patients (81%) received first-line antibiotics and, in contrast with other reports, receipt of first-line antibiotics did not vary by clinical location.16,27,28 Amoxicillin-clavulanate was the most commonly prescribed non-first-line antibiotic. In 23% of cases where amoxicillin-clavulanate was prescribed, the patient had concurrent conjunctivitis, for which use of amoxicillin-clavulanate is appropriate. Contrary to prior studies, we did not detect a difference in first-line prescribing between patients of different races or ethnicities.25,26
Despite current institutional guidelines recommending 5 days of antibiotics for children age ≥2 years with AOM, nearly all patients (94%) received antibiotic durations of >5 days, most patients (77%) received durations that exceeded prior institutional recommendations, and more than one-half of patients (55%) were prescribed ≥10 days of therapy. In our patient population, an antibiotic duration of 5 days, rather than ≥7 days, would have reduced days of antibiotic exposure by 41.3% (from 7762 to 4555 days) and an antibiotic duration of ≤7 days, rather than ≥10 days, would have decreased days of antibiotic exposure by 19.2% (from 7762 to 6269 days). Because AOM is the most common indication for antibiotics in children, a decrease in the duration of antibiotics prescribed would have a profound impact on overall antibiotic exposure for children. Thus, our leading priority for pediatric antibiotic stewardship has become decreasing the duration of therapy for AOM.
Numerous factors likely contribute to longer durations of antibiotics in our system. Currently in our EHR, only single-click options for antibiotic durations of 7 and 10 days are available and additional clicks are required to modify the antibiotic duration to 5 days. Although prescribing guidelines are available online and via a mobile app, clinical decision support is not directly available in the EHR. Finally, many providers continue to use 10 days of therapy for respiratory infections, potentially from extrapolation of streptococcus pharyngitis recommendations, lack of knowledge regarding current guidelines, or concern for lack of follow-up.
Several factors were associated with prolonged antibiotic courses. Patients who were younger and, thus, at greatest risk for disruption of the developing microbiome, were most likely to receive longer than recommended antibiotic courses.29 Although we did not detect differences in antibiotic duration by race, disparities in care were evident for different sexes and ethnicities. Patients who were non-Hispanic were more likely to receive prolonged antibiotic courses as well as females. Smith et al similarly found that in a Kentucky Medicaid population females were more likely to be prescribed antibiotics, although the reason for this disparity is unknown.30 We found that patients who were seen in the ED or urgent care were more likely to receive excessively long antibiotic courses compared with those seen in pediatric clinics. This finding parallels those from previous studies that have demonstrated that children managed in emergency departments are less likely to receive guideline-concordant treatment for common pediatric infections compared with those evaluated in outpatient clinics.28 Given this finding, our antibiotic stewardship interventions will target providers in all outpatient settings, including pediatric and nonpediatrics clinics as well as urgent cares and the emergency department.
The use of observation or SNAPs was exceedingly low. Immediate antibiotics were prescribed in 94% of cases. SNAPs and observation for AOM have shown to substantially reduce antibiotic use in children from 93% in children who receive an immediate prescription to 31%-45% for children who receive a SNAP and 14% in children who are initially managed by observation.10,31 However, given the challenges with distinguishing immediate vs SNAP prescriptions the national clinical prescribing rate of SNAPs for AOM are unknown.
Given that DH is a safety-net hospital and serves a predominantly high-risk Medicaid population, providers likely have enhanced concern for treatment failure and loss to follow-up, which could limit the use of observation and SNAPs. Transportation barriers and pharmacy access during non-DH pharmacy hours also limit the ability of patients to fill SNAPs or access care after failed observation if needed. Thus, although the use of SNAPs and observation has the ability to substantially decrease antibiotic use in many settings, broader systematic changes that also address social determinates of health are likely needed to improve SNAP and observation rates at DH. For some patients in our population, SNAPs or observation may simply not be practical or feasible. Additionally, challenges with EHR ordering of antibiotics likely contribute to low use of SNAPs in our system. Currently in our EHR, and we suspect in most EHR systems, antibiotic prescription orders do not have an efficient mechanism to differentiate an immediate prescription from a SNAP. Thus, providers must take additional time to send or print SNAPs. Changes to EHR systems to simplify ordering of SNAPs are needed. Such changes would also permit monitoring of SNAP prescribing rates to provide feedback to providers.
Our study has several strengths; the use of patient-level data permitted for an in-depth analysis of prescribing patterns. Although DH is university affiliated, it is predominantly a community-based integrated system and the patient population is likely similar to other urban health care systems.32 DH has a comprehensive EHR and patients largely use DH as their sole source of care, thus facilitating complete data capture. Additionally, we were able to evaluate prescribing patterns across a variety of clinical locations and provider types.
There are several important limitations to this study. First, despite manual chart abstraction we were unable to determine if patients diagnosed with AOM were diagnosed correctly or decipher the severity of infection. Second, we could not determine if patients filled or took antibiotics that were prescribed or for how long. This may have resulted in underreporting of SNAP prescribing. Third, because DH has a long-standing antimicrobial stewardship program and low comparative antibiotic use, the overuse of antibiotics for AOM demonstrated in this study may be an underrepresentation of antibiotic use for AOM at other institutions. The data presented here are from a single health care system and may not be generalizable to all institutions. However, the use of longer than recommended durations of antibiotics for other respiratory infections has been demonstrated on a national level, indicating that this is likely a ubiquitous problem.33,34 Finally, because we wanted to understand prescribing practices in the context of penicillin allergy and conjunctivitis and the number of patients who would have been excluded from these criteria was small (51 patients), we did not exclude them from the analysis. Had we excluded these patients, and the percent of patients who received first-line antibiotics would have been slightly higher (85.4%).
Given the magnitude of antibiotics prescribed for AOM, a variety of strategies are likely needed to reduce unnecessary antibiotic use. Although most antimicrobial stewardship interventions for AOM have focused on improving first-line antibiotic use, our data suggest that an emphasis on the duration of antibiotic therapy as well as the use of SNAPs or observation may be higher yield targets. The considerable variability in prescribing patterns between provider types presented here and in other studies suggests that antimicrobial stewardship interventions targeting a broad range of provider types, rather than pediatricians alone, are vital to improving overall antibiotic use for AOM in children.
Acknowledgments
H.M.F. received salary support from the Eunice Kennedy Shriver National Institute Of Child Health & Human Development of the National Institutes of Health under Award Number K23HD099925. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Glossary
- AOM
Acute otitis media
- DH
Denver Health
- EHR
Electronic health record
- SNAP
Safety net antibiotic prescription
Footnotes
Portions of this study were presented at the 10th Annual Pediatric Antimicrobial Stewardship Conference, 30-31, 2019, St. Louis, Missouri.
References
- 1.Hersh AL, Shapiro DJ, Pavia AT, Shah SS. Antibiotic prescribing in ambulatory pediatrics in the United States. Pediatrics 2011;128:1053–61. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Antibiotic resistance threats in the united states, 2013. https://www.cdc.gov/drugresistance/pdf/arthreats-2013-508.pdf. Accessed December 3, 2013.
- 3.Gerber JS, Ross RK, Bryan M, Localio AR, Szymczak JE, Wasserman R, et al. Association of broad- vs narrow-spectrum antibiotics with treatment failure, adverse events, and quality of life in children with acute respiratory tract infections. JAMA 2017;318:2325–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Horton DB, Scott FI, Haynes K, Putt ME, Rose CD, Lewis JD, et al. Antibiotic exposure and juvenile idiopathic arthritis: a case-control study. Pediatrics 2015;136:e333–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kronman MP, Zaoutis TE, Haynes K, Feng R, Coffin SE. Antibiotic exposure and IBD development among children: a population-based cohort study. Pediatrics 2012;130:e794–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wendt JM, Cohen JA, Mu Y, Dumyati GK, Dunn JR, Holzbauer SM, et al. Clostridium difficile infection among children across diverse US geographic locations. Pediatrics 2014;133:651–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shaikh N, Dando EE, Dunleavy ML, Curran DL, Martin JM, Hoberman A, et al. A cost-utility analysis of 5 strategies for the management of acute otitis media in children. J Pediatr 2017;189:54–60.e3. [DOI] [PubMed] [Google Scholar]
- 8.Kaur R, Morris M, Pichichero ME. Epidemiology of Acute Otitis Media in the Postpneumococcal Conjugate Vaccine Era. Pediatrics 2017;140:e20170101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Venekamp RP, Sanders SL, Glasziou PP, Del Mar CB, Rovers MM. Antibiotics for acute otitis media in children. Cochrane Database Syst Rev 2015;6:Cd000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spurling GK, Del Mar CB, Dooley L, Foxlee R, Farley R. Delayed antibiotic prescriptions for respiratory infections. Cochrane Database Syst Rev 2017;9:Cd004417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lieberthal AS, Carroll AE, Chonmaitree T, Ganiats TG, Hoberman A, Jackson MA, et al. The diagnosis and management of acute otitis media. Pediatrics 2013;131:e964–99. [DOI] [PubMed] [Google Scholar]
- 12.National Institute for Health and Care Excellence. Otitis media (acute): antimicrobial prescribing NICE Guideline, 2018. www.nice.org.uk/guidance/ng91 Accessed September 2, 2018.
- 13.Greenberg D, Givon-Lavi N, Sadaka Y, Ben-Shimol S, Bar-Ziv J, Dagan R. Short-course antibiotic treatment for community-acquired alveolar pneumonia in ambulatory children: a double-blind, randomized, placebo-controlled trial. Pediatr Infect Dis J 2014;33:136–42. [DOI] [PubMed] [Google Scholar]
- 14.Haider BA, Saeed MA, Bhutta ZA. Short-course versus long-course antibiotic therapy for non-severe community-acquired pneumonia in children aged 2 months to 59 months. Cochrane Database Syst Rev 2008;2:Cd005976. [DOI] [PubMed] [Google Scholar]
- 15.Kozyrskyj A, Klassen TP, Moffatt M, Harvey K. Short-course antibiotics for acute otitis media. Cochrane Database Syst Rev 2010;9:Cd001095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frost HM, McLean HQ, Chow BDW. Variability in antibiotic prescribing for upper respiratory illnesses by provider specialty. J Pediatr 2018;203:76–85.e8. [DOI] [PubMed] [Google Scholar]
- 17.Gerber JS, Prasad PA, Russell Localio A, Fiks AG, Grundmeier RW, Bell LM, et al. Variation in antibiotic prescribing across a pediatric primary care network. J Pediatr Infect Dis Soc 2015;4:297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hersh AL, Fleming-Dutra KE, Shapiro DJ, Hyun DY, Hicks LA. Frequency of first-line antibiotic selection among US ambulatory care visits for otitis media, sinusitis, and pharyngitis. JAMA Intern Med 2016;176:1870–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gerber JS, Prasad PA, Fiks AG, Localio AR, Grundmeier RW, Bell LM, et al. Effect of an outpatient antimicrobial stewardship intervention on broad-spectrum antibiotic prescribing by primary care pediatricians: a randomized trial. JAMA 2013;309:2345–52. [DOI] [PubMed] [Google Scholar]
- 20.Spellberg B The new antibiotic mantra-“shorter is better.” JAMA Intern Med 2016;176:1254–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Health Denver and Authority Hospital. Report to the city. Denver, CO: Denver Health, 2018. https://www.denverhealth.org/-/media/files/about/annual-reports/gr1902-07-reporttothecity-2018-final-web.pdf?la=en&hash=E4323D81B05197C29485D4C74A3C9466FFE42042 Accessed October 1, 2019.
- 22.Health Resources and Services Administration. Denver Health & Hospital Authority Health Center Program Awardee Data. 2018. https://bphc.hrsa.gov/uds/datacenter.aspx?q=d&bid=080060&state=CO&year=2018 Accessed December 3, 2019.
- 23.Jenkins TC, Knepper BC, Shihadeh K, Haas MK, Sabel AL, Steele AW, et al. Long-term outcomes of an antimicrobial stewardship program implemented in a hospital with low baseline antibiotic use. Infect Control Hosp Epidemiol 2015;36:664–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Young HL, Shihadeh KC, Skinner AA, Knepper BC, Sankoff J, Voros J, et al. Implementation of an institution-specific antimicrobial stewardship smartphone application. Infect Control Hosp Epidemiol 2018;39:986–8. [DOI] [PubMed] [Google Scholar]
- 25.Fleming-Dutra KE, Shapiro DJ, Hicks LA, Gerber JS, Hersh AL. Race, otitis media, and antibiotic selection. Pediatrics 2014;134:1059–66. [DOI] [PubMed] [Google Scholar]
- 26.Gerber JS, Prasad PA, Localio AR, Fiks AG, Grundmeier RW, Bell LM, et al. Racial differences in antibiotic prescribing by primary care pediatricians. Pediatrics 2013;131:677–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hicks LA, Bartoces MG, Roberts RM, Suda KJ, Hunkler RJ, Taylor TH Jr, et al. US outpatient antibiotic prescribing variation according to geography, patient population, and provider specialty in 2011. Clin Infect Dis 2015;60:1308–16. [DOI] [PubMed] [Google Scholar]
- 28.Agiro A, Gautam S, Wall E, Hackell J, Helm M, Barron J, et al. Variation in outpatient antibiotic dispensing for respiratory infections in children by clinician specialty and treatment setting. Pediatr Infect Dis J 2018;37:1248–54. [DOI] [PubMed] [Google Scholar]
- 29.Gibson MK, Crofts TS, Dantas G. Antibiotics and the developing infant gut microbiota and resistome. Current opinion in microbiology 2015;27: 51–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poster Abstract. IDWeek New Orleans, LA October 29, 2019. https://idsa.confex.com/idsa/2016/webprogram/Paper57525.html. Accessed February 9, 2020.
- 31.Siegel RM, Kiely M, Bien JP, Joseph EC, Davis JB, Mendel SG, et al. Treatment of otitis media with observation and a safety-net antibiotic prescription. Pediatrics 2003;112:527–31. [DOI] [PubMed] [Google Scholar]
- 32.Health Resources and Services Administration. Denver Health & Hospital Authority Health Center Program Awardee Data; 2018. https://bphc.hrsa.gov/uds/datacenter.aspx?q=d&bid=080060&state=CO&year=2018 Accessed December 3, 2019.
- 33.King LM, Sanchez GV, Bartoces M, Hicks LA, Fleming-Dutra KE. Antibiotic therapy duration in US adults with sinusitis. JAMA Intern Med 2018;178:992–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yi SH, Hatfield KM, Baggs J, Hicks LA, Srinivasan A, Reddy S, et al. Duration of antibiotic use among adults with uncomplicated community-acquired pneumonia requiring hospitalization in the United States. Clin Infect Dis 2018;66:1333–41. [DOI] [PMC free article] [PubMed] [Google Scholar]


