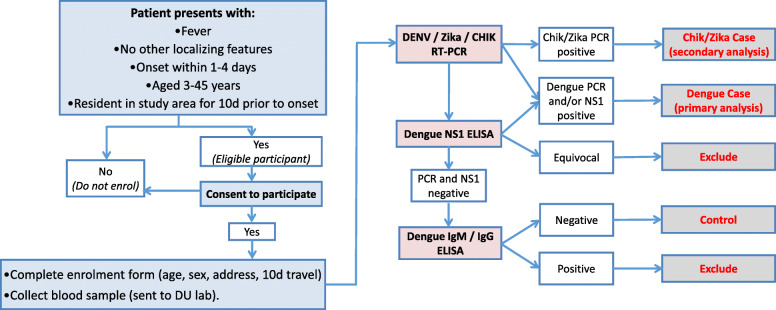
Fig. 1.
Flowchart of data and sample collection procedures and diagnostic algorithm. Blue boxes indicate participant recruitment and enrolment activities undertaken at Puskesmas clinics, including screening against inclusion/exclusion criteria, obtaining written informed consent, and collection of demographic and travel history data and a blood sample. Pink boxes indicate the laboratory diagnostic testing to be performed at the project laboratory (DU), the results of which (white boxes) will be used to classify participants as virologically confirmed dengue, Zika or chikungunya (Chik) cases or arbovirus-negative controls or excluded because of an inability to exclude arbovirus infection (grey boxes) according to the algorithm shown. This diagnostic algorithm is updated from the original version to indicate that all samples are tested by both reverse transcriptase polymerase chain reaction (RT-PCR) and dengue virus (DENV) non-structural protein 1 (NS1) enzyme-linked immunosorbent assay (ELISA). In the original algorithm, only samples that were PCR-negative for dengue, chikungunya and Zika were tested by NS1 ELISA