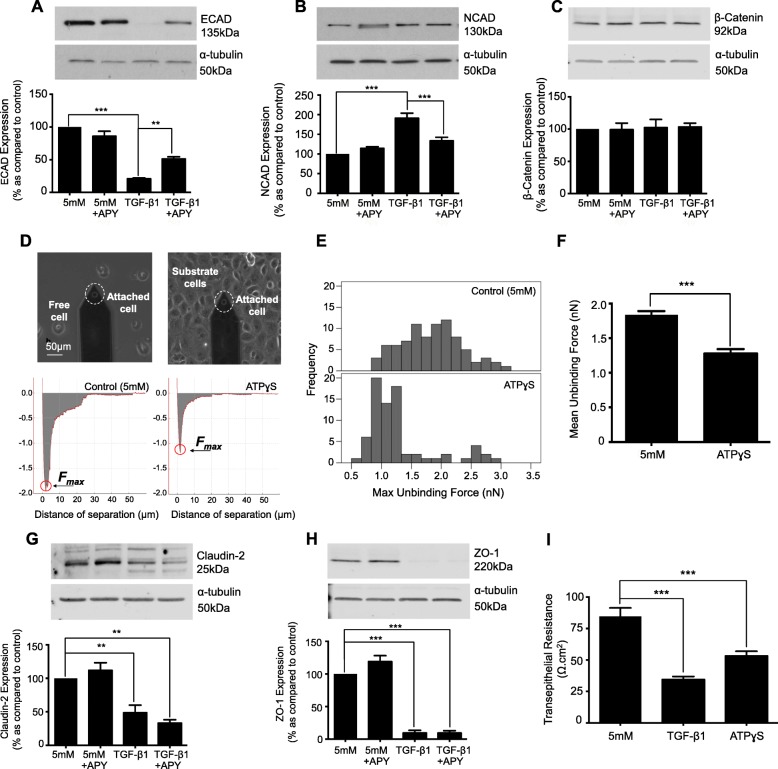
Fig. 3.
The ATP-diphosphohydrolase, apyrase negates TGF-β1-evoked changes in expression of the adherens junction complex, but fails to restore TGF-β1 induced changes in tight junction protein expression. To determine whether ATP mediates TGF-β1-evoked changes in the expression of adherens and tight junction proteins, cells were cultured in low glucose (5mM) ± TGF-β1 (10 ng/mL) ± apyrase (5 U/ml) for 48 h. Whole-cell expression of E-cadherin (a), N-cadherin (b), β-catenin (c), Claudin-2 (g), and ZO-1 (h) were assessed via immunoblotting. Representative blots for each protein are shown, with expression normalized by re-probing for ɑ-tubulin as a loading control. Bars correspond to their associated lanes in the respective blot. Changes in expression were matched to changes in function (d-f) and (i). Atomic Force Microscopy single-cell force spectroscopy was used to measure the maximum unbinding force required to uncouple two adhered cells. Human renal tubule cells were cultured in low glucose with/without ATPγS (100 μM) for 48 h. Retraction Force-displacement curves for control and ATPγS (d) cells are shown respectively. Maximum unbinding forces (e & f) between two cells was determined by measuring the amplitude of the points circled in red (d). Lastly, disassembly of the tight junction complex is paralleled by loss of transepithelial electrical resistance (TER). Cells were cultured in low glucose ± either TGF-β1 (10 ng/mL) or ATPγS (100 μM) for 48 h on Transwell inserts. Data is expressed as mean ± SEM of multiple cells from 4 separate experiments, with key significance shown: **P < 0.01, ***P < 0.001