Figure 1. Protein synthesis by ligation techniques.
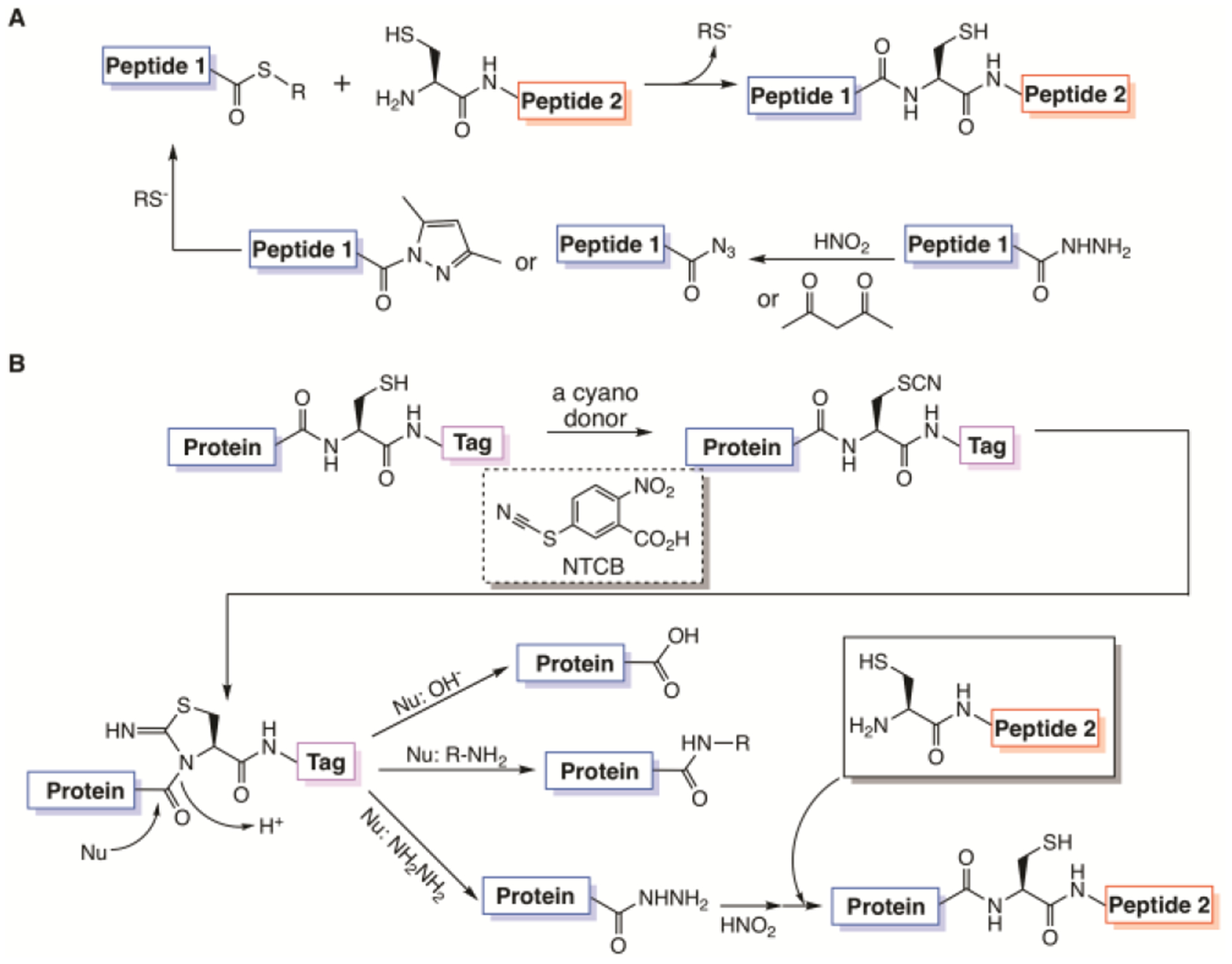
(A) Native chemical ligation and a derivative technique, peptide hydrazide ligation; (B) A proposed ACPL technique based on nucleophilic acyl substitution of an activated cysteine residue in a recombinant protein by a nucleophilic amine. Without a nucleophilic amine, the protein undergoes hydrolysis. When the nucleophile is hydrazine, the afforded protein hydrazide can then undergo peptide hydrazide ligation to form a larger protein.
