Figure 3. The synthesis of FLAG-Ub/Ubl-Pa and Ub/FLAG-SUMO1–3-AMC probes by ACPL and their applications in covalent conjugation or activity assays of DUB/ULPs.
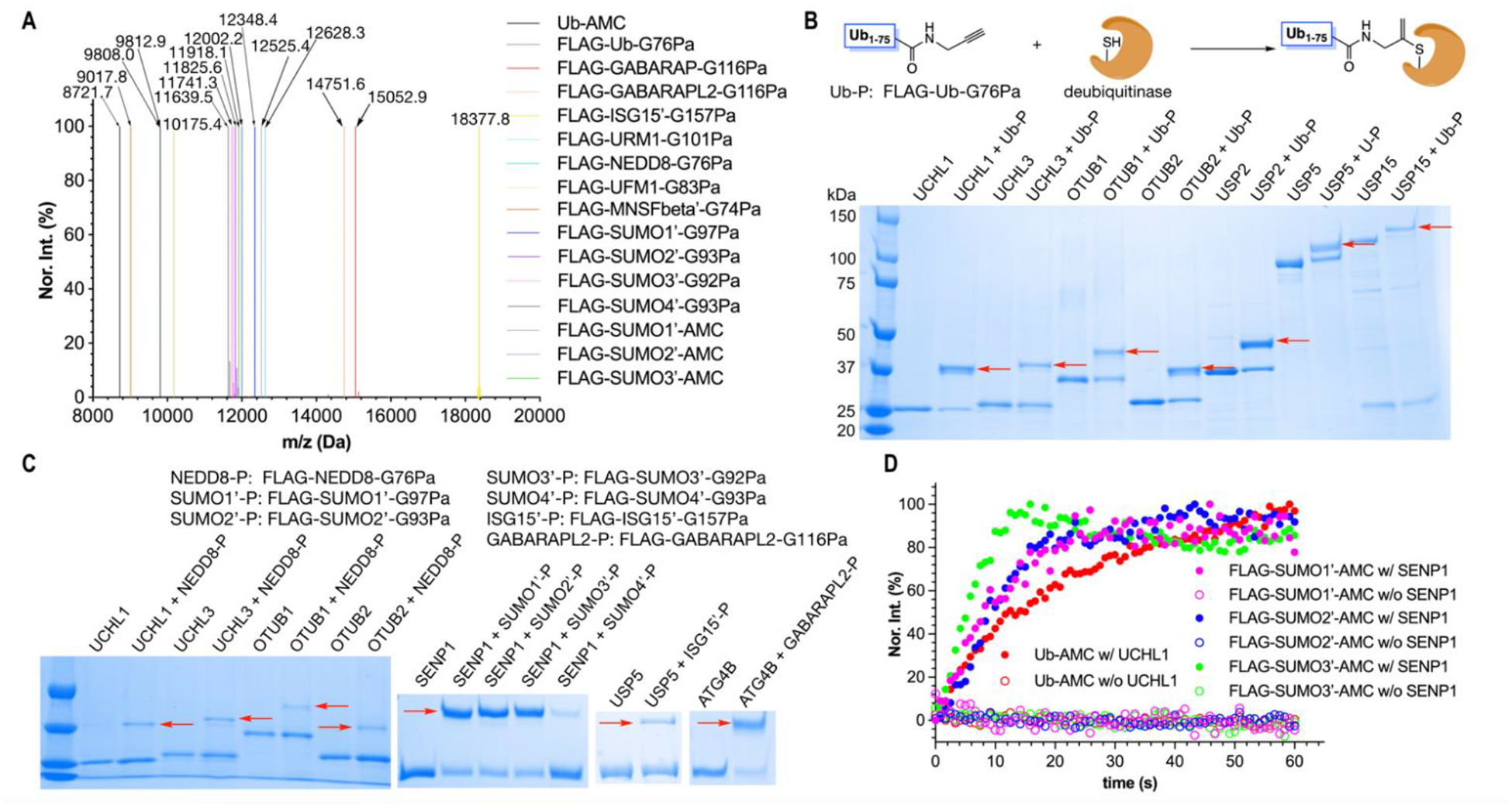
(A) The deconvoluted and integrated ESI-MS of FLAG-Ub/Ubl-Pa and Ub/FLAG-SUMO1–3-AMC probes. Ub-AMC was synthesized from Ub-G76C-6H. All other Pa- and AMC-conjugated Ub/Ubls were generated from FLAG-tagged proteins. Ub/Ubls with their C-terminal glycine mutated to cysteine were expressed and purified as a protein fused with a N-terminal FLAG tag and a C-terminal 6×His tag. ISG15, SUMO1–4, and MNSF have a native cysteine residue. This cysteine was mutated to alanine or serine in all six expressed proteins for avoiding side reactions. The label “′” indicates this mutation. All detected molecular weights agreed well with their theoretic values with a deviation range of 0.5 Da. (B) The formation of covalent adducts between FLAG-Ub-G76Pa and a number of DUBs. Red arrows point to the generated adducts. (C) The formation of covalent adducts, indicated by red arrows, between different FLAG-Ubl-GxPa probes and DUB/ULPs. (D) The DUB/ULP-catalyzed AMC release from Ub-AMC and three FLAG-SUMO-AMC conjugates.
