Abstract
As an antagonist for the WNT signal passway, dickkopf-1(DKK1) have a great important role in the occurence and development of various type cancer. The present paper performed a meta-analysis to evaluate the predictive significance of DKK1 in cancer.
To assess the relationship between the expression of DKK1 and prognostic role in human cancers, a total of 16 articals were screened from the multiple online databases (Pubmed, EMBASE, CNKI, Web of Science and Google Scholar) in our study. By using the STATA soft,pooled hazard ratio and 95% confidence intervals of overall survival (OS), progression-free survival, disease-free survival and time to recurrence were used to evaluate the strength of this relationship.
The meta-analysis showed that higher expression of DKK1 was significantly associated with shorter OS in cancer patients. In stratified analyzes, the higher expression of DKK1 could reduced the OS in patients with breast cancer,digestive system cancer and urogenital system cancer, but not patients with the lung cancer. It also showed that higher expression of DKK1 was significantly associated with shorter progression-free survival, disease-free survival and time to recurrence in cancer patients.
The present study indicate that higher expression of DKK1 predict an unfavorable clinical outcome in patients with breast cancer, digestive system cancer and urogenital system cancer.
Keywords: cancer, dickkopf-1, meta-analysis
1. Introduction
With the change of people's living habits and the deterioration of the environment, the incidence of cancer is increasing year by year. Although the detection methods have been improved a lot, most of the tumors are diagnosed at an advanced stage, resulting in a high mortality rates. Prognostic assessment can help doctors understand their patient's condition and follow-up development. In order to improve the survival time of patients, we can select appropriate targets and treatment methods through the study of prognostic diagnostic indicators and treatment methods. Research on markers related to prognosis of tumors is in full swing.
Dickkopf-1 (DKK1) is a secretory protein, which is a member of DKK family.[1] WNT /β-catenin pathway is a group of signal transduction pathway which plays a fundamental role in cell growth and development. When the WNT pathway is activated abnormally, the nuclear regulator β-catenin is increased, which is involved in the transcription of genetic information and induces many kinds of tumors.DKK1 can prevent Wnt signaling pathway from transmitting to Intracellular through combining with low density lipoprotein receptor-related protein LRP5/LRP6.[2,3] However,dkk1 was found to be highly or poorly expressed in various tumors, showing a variety of characteristics, such as high expression[4–6] in non-small cell lung cancer, liver cancer and esophageal cancer, while low expression[7–9] in colon cancer, renal cell cancer and leukemia. The main reason is that DKK1 plays different roles in different tumors and stages.
DKK1 is expressed differently in different tumors in the current published articles, and it is on this basis that we would like to find out whether its prognostic effects are consistent for tumors. This paper intends to explore the role of DKK1 in the prognosis of cancer by enlarging the research number and sample size, then making a meta-analysis on it.
2. Methods
2.1. Search strategy
In order to evaluate the relationship between the expression of DKK1 and survival time in the patients with cancer,relative articles were searched from these online databases (Pubmed, EMBASE, CNKI, Web of Science and Google Scholar). The keywords for the search were: (DKK1 [Title/Abstract] OR DKK-1 [Title/Abstract] OR dickkopf-1 [Title/Abstract]) AND (neoplasms [Title/Abstract] OR tumor [Title/Abstract] OR cancer [Title/Abstract] OR carcinoma [Title/Abstract]) AND (prognosis [Title/ Abstract] OR survival [Title/Abstract]).
2.2. Inclusion and exclusion criteria
The selected study should meet the following Inclusion criteria:
-
(1)
must be the original articles;
-
(2)
Patients in the study were definitely diagnosed as cancer;
-
(3)
Patients were divided into 2 groups according to the level of DKK1 expression;
-
(4)
associations of DKK1 expression levels with overall survival (OS), progression-free survival (PFS), DFS, and time to recurrence (TTR) were described;
-
(5)
hazard ratios (HRs) with 95% confidence of the OS, PFS, DFS, and TTR must be displayed directly or calculated indirectly by the author; and
-
(6)
study should be published in English and must have the full text.
Exclusion criteria for the articles included:
-
(1)
Study have no complete data, unable to extract relevant data for us;
-
(2)
duplicated publications;
-
(3)
were not published as research articles including summaries, commentary, case reports, letters or non-case-control studies.
2.3. Endpoints
In this article, we defined OS as the primary endpoint; PFS, DFS, and TTR as the secondary endpoint. OS refers to the period from the beginning of curative operation to the end of the last follow-up or death of any cause. While the PFS was the time from the beginning of curative operation to the progression of tumors or the death of any cause, DFS to the recurrence of cancer or death,TTR was The time from CR or PR to PD (Progressive Disease) or any cause of death.
2.4. Data extraction and quality assessment
The following information were extracted from all included eligible studies independently by 2 authors (WB K and JF H):the name of first author, year of publication, cancer type, clinical Stage,total number of patients, outcome measures, the patient numbers of high/low DKK1 expression, year of survival and HR and its corresponding 95% confidence intervals (CI). Enguage Digitizer (Version 4.1) software were used to extract the data from the papers only have Kaplan-Meier curves. We would rather choose multivariate analysis than univariate ones because multivariate analysis have more accurate statistical significance. When inconsistencies arise, a third person (T L) will evaluate again.
The Quality of the studies were assessed by the Newcastle-Ottawa Scale (NOS), The score of NOS scoring system ranged from 0 to 9, more than 6 points are of high quality.
2.5. Statistical analysis
The meta-analysis was performed by the software STATA 15.0. The heterogeneity between studies were assessed by the chi-squared based Q-test or I2 test. P < .05 for the Q test (Ph) and I2 > 50% were considered to be significantly heterogeneous. When heterogeneity occurs, the random effect model is used, otherwise the fixed effect models is used. Begg funnel plot and Egger test were applied to checkout the potential publication bias. The sensitivity analysis was used to judge the stability of the results. P < .05 was considered to be statistically significant.
2.6. Medical ethics
The ethical approval in this paper was not necessary because it is a Meta analysis, so there is no need to deal with ethical issues.
3. Results
3.1. Studies searching results and characteristics of eligible studies
We finally chose 16 studies about the relationship between the expression of DKK1 and survival time in the patients with cancer which all meet the inclusion criteria. The whole screening process was shown in the Figure 1. All the acquired papers were calculated by the NOS. Characteristics of included studies were shown in the Table 1.
Figure 1.
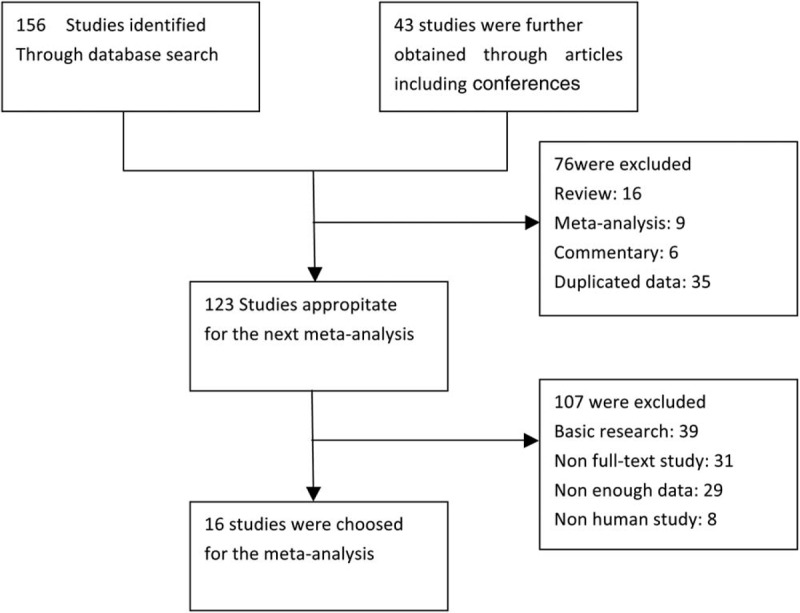
Flow chart diagram of selecting eligible studies for meta-analysis.
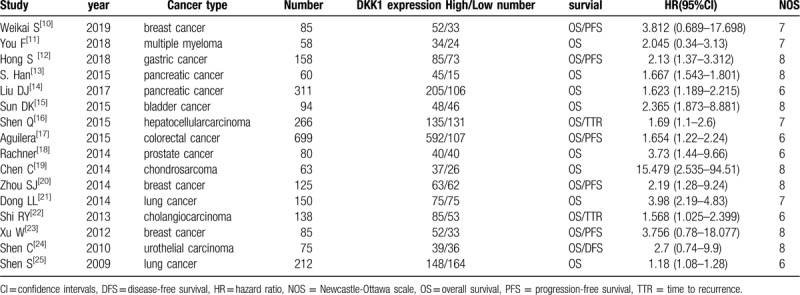
Table 1.
Main characteristics of included eligible studies.
3.2. Meta-analysis of the association between the expression of DKK1 and OS
The random-effects model was used in this Meta-analysis because of the heterogeneity test (I2 = 81.7%, P < .001).It showed that higher expression of DKK1 was significantly associated with shorter OS in cancer patients (HR = 1.96, 95% CI 1.61-2.37, P < .001). (Fig. 2).
Figure 2.
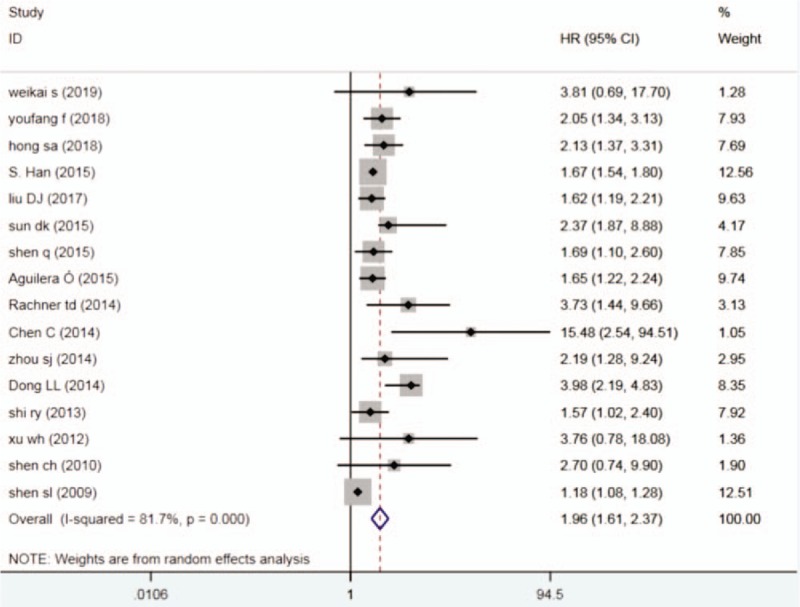
Forest plots describing HR of the association between DKK1 expression and OS in human tumors from 16 papers, DKK1 = dickkopf-1, HR = hazard ratio, OS = overall survival.
In stratified analyses according to cancer types,the higher expression of DKK1 could reduce the OS in patients with breast cancer (HR = 1.96, 95% CI 1.61-2.37, P = .007),digestive system cancer (HR = 1.67,95% CI 1.56-1.79, P < .001)and urogenital system cancer (HR = 2.81,95%CI 1.63-7.486, P < .001),but not patients with the lung cancer (Fig. 3).
Figure 3.
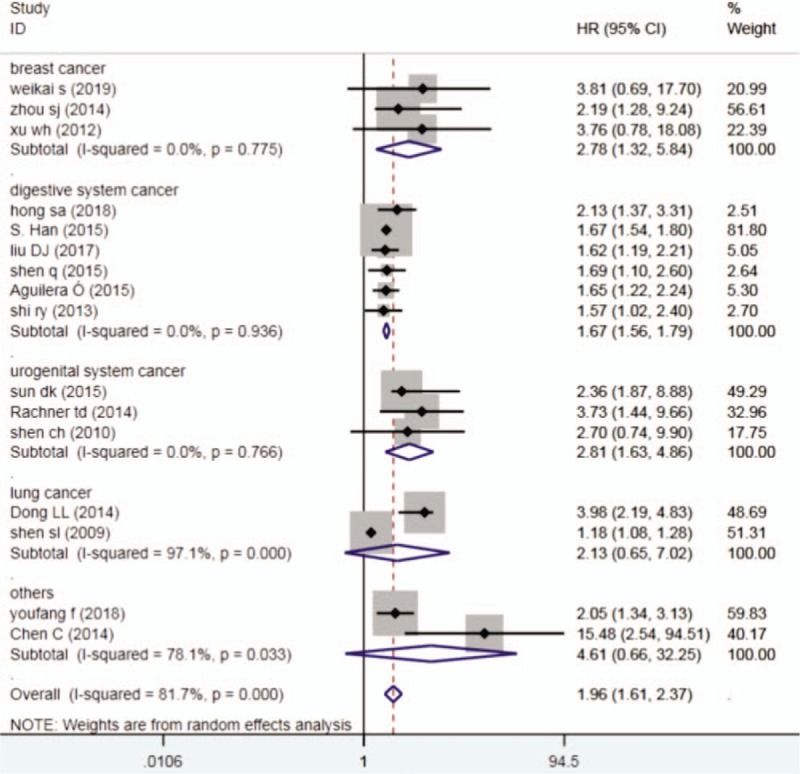
Forest plots of subgroup analysis (cancer type) describing HR of the association between DKK1 expression and OS in human tumors, DKK1 = dickkopf-1, HR = hazard ratio, OS = overall survival.
3.3. Meta-analysis of the association between the expression of DKK1 and PFS
Meta-analysis also showed that higher expression of DKK1 was significantly associated with shorter PFS in patients with breast cancer, gastric cancer and colorectal cancer (HR = 1.89,95% CI 1.56-2.30, P < .001) (Fig. 4).
Figure 4.
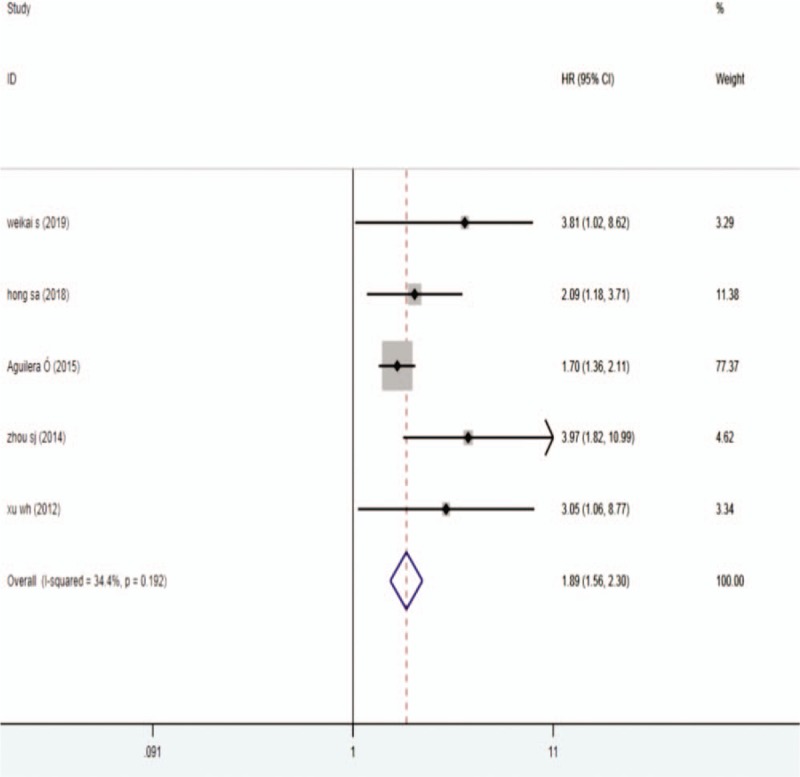
Forest plots describing HR of the association between DKK1 expression and PFS in human tumors from 5 papers, DKK1 = dickkopf-1, HR = hazard ratio, PFS = progression-free survival.
3.4. Meta-analysis of the association between the expression of DKK1 and disease-free survival (DFS) and TTR
The results indicated that the higher DKK1 expression level group can reduce the TTR in cancer patients(HR = 2.02,95% CI 1.51-2.71, P < .001) and the time to DFS (HR = 2.36,95% CI 1.06-5.25, P = .035) (Fig. 5).
Figure 5.
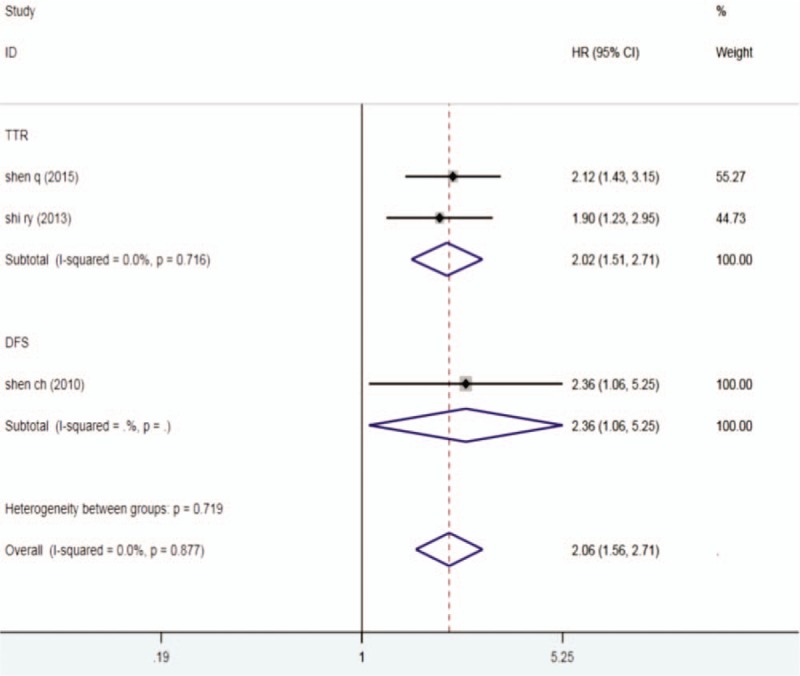
Forest plots describing HR of the association between DKK1 expression and TTR and DFS in human tumors from 3 papers, DKK1 = dickkopf-1, HR = hazard ratio, DFS = disease-free survival, TTR = time to recurrence.
3.5. Sensitivity analysis and publication bias
The results showed that each result had no significant effect on overall HR for OS, PFS, TTR, and DFS. According to Funnel plot and Egger test,there was no publication bias in our papers between DKK1 expression and OS (P = .392) or PFS (P = .07).
4. Discussion
The human cancer has the following characteristics: high morbidity, high mortality, low early detection rate and low survival rate. Despite the large amount of funds and money in recent years are used to support cancer research about diagnosis and treatment, the effect is not as good as expected. The Oncology research has a long way to go. We must go ahead bravely.
DKK1, 1 of the DKK family genes, inhibits WNT signaling pathway through 2 mechanisms: binding with low density lipoprotein receptor related proteins (LRP5/LRP6) and Kremen1, Kremen2, which are the co-receptor of WNT, then inducing rapid endocytosis, reducing the level of LRP5/LRP6 on the cell membrane, thus blocking Wnt signal transmission to the Intracellular;[26] Besides, DKK1 could block the formation of WNT-guided LRP6 complex by connecting with LRP6.[27] In many tumors, Wnt pathway is over-activated. DKK-1, as a classical Wnt Pathway inhibitors, have also attracted much attentions, and more and more studies have been carried out on them.
In previous studies, DKK1 was found to be highly or poorly expressed in various tumors, showing a variety of characteristics, such as high expression[4–6] in non-small cell lung cancer, liver cancer and esophageal cancer, while low expression[7–9] in colon cancer, renal cell cancer and leukemia. It is associated with the invasion and metastasis of most tumors, but not with the proliferation of tumor cells. It is 1 of the new indicators for the diagnosis of potential malignant tumors and may be 1 of the potential therapeutic targets for malignant tumors. Most studies have shown that its expression level is correlated with survival.
In our meta-analysis, our main aim was to analyze the correlation between DKK1 expression and prognosis of tumors. As described in the above results, the higher the level of DKK-1, the shorter the OS, PFS, DFS, and TTR in cancer patients. This indicates that DKK1 predict poor prognosis in most human tumors.
In stratified analyses, the higher expression of DKK1 could reduced the OS in patients with breast cancer, digestive system cancer and urogenital system cancer,but not patients with the lung cancer. But in perious papers, DKK1 is highly expressed in the serum of lung cancer patients. Overexpression of DKK1 promotes migration and invasion of lung cancer cells.[28] On metastasis of tumors, complex and diverse research results have emerged in different tumors and microenvironments: Serum DKK1 concentration in patients with non-bone metastasis of non-small cell lung cancer was significantly higher than that in patients with bone metastasis.[29] However, a recent study[30] by the Chinese Academy of Sciences showed that breast cancer patients with higher DKK1 secretion tended to metastasize to bone, while those with lower DKK1 secretion tended to metastasize to lung. These different findings suggest that there are many unknown areas of DKK1 expression in lung cancer, waiting for us to uncover.
There are still some limitations in this meta-analysis. First, the total number of studies included is still small. Second, there is no uniform standard for cut-off values from different research methods. Third, There are only 2 studies on the relationship between DKK1 and TTR in cancer patients, and only 1 study reporting the relationship between DKK1 and DFS, we couldn’t get a better pooled result for them. Finally, unpublished studies with negative results may lead to potential publication bias.
In summary, our study showed that cancer patients with higher DKK1 expression may have a shorter survival time. DKK1 can be used as a new target for diagnosis and treatment of tumors. These conclusions need a lot of clinical and basic experiments to verify.
Author contributions
JF H and T L conceived and designed this study;
JF H and WB K searched databases and collected the data;
JF H performed the statistical analysis;
T L drafted the manuscript. All authors reviewed the final manuscript.
Footnotes
Abbreviations: CI = confidence intervals, DFS = disease-free survival, DKK1 = dickkopf-1, HR = hazard ratio, OS = overall survival, PFS = progression-free survival, TTR = time to recurrence.
How to cite this article: Huang J, Lu T, Kuang W. Prognostic role of dickkopf-1 in patients with cancer. Medicine. 2020;99:21(e20388).
JH and TL authors contributed equally to this study.
Research was supported by the National Natural Science Foundation of China (81601382), Scientific research project of traditional Chinese medicine in Guangdong Province(20172122), Zhejiang Medical Science and Technology Program (2020RC130), Shaoxing People's Hospital Youth Fund(2018YB02)
The authors have no funding and conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Park BM, Kim EJ, Nam HJ, et al. Cyclized oligopeptide targeting LRP5/6-DKK1 interaction reduces the growth of tumor burden in a multiple myeloma mouse model. Yonsei Med J 2017;58:505–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Shangguan L, Ning G, Luo Z, et al. Fibulin-4 reduces extracellular matrix production and suppresses chondrocyte differentiation via DKK1- mediated canonical Wnt/β-catenin signaling. Int J Biol Macromol 2017;99:293–9. [DOI] [PubMed] [Google Scholar]
- [3].Essa ES, Montaser BA, Badawy MT, et al. DKK1 in relation to HCV induced liver cirrhosis and HCV induced HCC curative resection. Acta Gastroenterol Belg 2016;79:309–13. [PubMed] [Google Scholar]
- [4].Xiang XJ, Liu YW, Chen DD, et al. Differential expression of Dickkopf-1 among non-small cell lung cancer cells. Mol Med Rep 2015;12:1935–40. [DOI] [PubMed] [Google Scholar]
- [5].Li J, Gong W, Li X, et al. Recent progress of Wnt pathway inhibitor dickkopf-1 in liver cancer. J Nanosci Nanotechnol 2018;18:5192–206. [DOI] [PubMed] [Google Scholar]
- [6].Lyros O, Lamprecht AK, Nie L, et al. Dickkopf-1 (DKK1) promotes tumor growth via Akt-phosphorylation and independently of Wnt-axis in Barrett's associated esophageal adenocarcinoma. Am J Cancer Res 2019;9:330–46. [PMC free article] [PubMed] [Google Scholar]
- [7].Jia Y, Chen L, Guo S, et al. Baicalin induced colon cancer cells apoptosis through miR-217/DKK1-mediated inhibition of Wnt signaling pathway. Mol Biol Rep 2019;46:1693–700. [DOI] [PubMed] [Google Scholar]
- [8].Guo CC, Zhang XL, Yang B, et al. Decreased expression of Dkk1 and Dkk3 in human clear cell renal cell carcinoma. Mol Med Rep 2014;9:2367–73. [DOI] [PubMed] [Google Scholar]
- [9].Firtina S, Hatirnaz Ng Ö, Erbilgin Y, et al. Dysregulation of the DKK1 gene in pediatric B-cell acute lymphoblastic leukemia. Turk J Med Sci 2017;47:357–63. [DOI] [PubMed] [Google Scholar]
- [10].Weikai s, Shang J, Zhang J, et al. Correlations of DKK1 with incidence and prognosis of breast cancer. J BUON 2019;24:26–32. [PubMed] [Google Scholar]
- [11].Youfang f, Zhang Y, Wei X, et al. Correlations of DKK1 with pathogenesis and prognosis of human multiple myeloma. Cancer Biomark 2019;24:195–201. [DOI] [PubMed] [Google Scholar]
- [12].Hong SA, Yoo SH, Lee HH, et al. Prognostic value of Dickkopf-1 and ß-catenin expression in advanced gastric cancer. BMC Cancer 2018;18:506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Han S, Zhou X, Sui X, et al. Serum dickkopf-1 is a novel serological biomarker for the diagnosis and prognosis of pancreatic cancer. Oncotarget 2015;6:19907–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Liu DJ, Xie YX, Liu XX, et al. The role of Dickkopf-1 as a potential prognostic marker in pancreatic ductal adenocarcinoma. Cell Cycle 2017;16:1622–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Sun DK, Wang L, Wang JM, et al. Serum Dickkopf-1 levels as a clinical and prognostic factor in patients with bladder cancer. Genet Mol Res 2015;14:18181–7. [DOI] [PubMed] [Google Scholar]
- [16].Shen Q, Yang XR, Tan Y, et al. High level of serum protein DKK1 predicts poor prognosis for patients with hepatocellular carcinoma after hepatectomy. Hepat Oncol 2015;2:231–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Aguilera Ó, González-Sancho JM, et al. Nuclear DICKKOPF-1 as a biomarker of chemoresistance and poor clinical outcome in colorectal cancer. Oncotarget 2015;6:5903–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Rachner TD, Thiele S, Göbel A, et al. High serum levels of Dickkopf-1 are associated with a poor prognosis in prostate cancer patients. BMC Cancer 2014;14:649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Chen C, Zhou H, Zhang X, et al. Elevated levels of Dickkopf-1 are associated with (-catenin accumulation and poor prognosis in patients with chondrosarcoma. PLoS One 2014;9:e105414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Zhou SJ, Zhuo SR, Yang XQ, et al. Serum Dickkopf-1 expression level positively correlates with a poor prognosis in breast cancer. Diagn Pathol 2014;9:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Dong LL, Qu LY, Chu LY, et al. Serum level of DKK-1 and its prognostic potential in non-small cell lung cancer. Diagn Pathol 2014;9:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Shi RY, Yang XR, Shen QJ, et al. High expression of Dickkopf-related protein 1 is related to lymphatic metastasis and indicates poor prognosis in intrahepatic cholangiocarcinoma patients after surgery. Cancer 2013;119:993–1003. [DOI] [PubMed] [Google Scholar]
- [23].Xu WH, Liu ZB, Yang C, et al. Expression of dickkopf-1 and beta-catenin related to the prognosis of breast cancer patients with triple negative phenotype. PLoS One 2012;7:e37624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Shen CH, Hsieh HY, Wang YH, et al. High Dickkopf-1 expression is associated with poor prognosis in patients with advanced urothelial carcinoma. Exp Ther Med 2010;1:893–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Sheng SL, Huang G, Yu B, et al. Clinical significance and prognostic value of serum Dickkopf-1 concentrations in patients with lung cancer. Clin Chem 2009;55:1656–64. [DOI] [PubMed] [Google Scholar]
- [26].Zhang P, Li S, Lv C, et al. BPI-9016 M, a c-Met inhibitor, suppresses tumor cell growth, migration and invasion of lung adenocarcinoma via miR203-DKK1. Theranostics 2018;8:5890–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kwack MH, Lee JH, Seo CH, et al. Dickkopf-1 is involved in dexamethasone-mediated hair follicle regression. Exp Dermatol 2017;26:952–4. [DOI] [PubMed] [Google Scholar]
- [28].Han S, Yang X, Pan Y, et al. L-securinine inhibits the proliferation of A549 lung cancer cells and promotes DKK1 promoter methylation. Oncol Lett 2017;14:4243–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Chu T, Teng J, Jiang L, et al. Lung cancer-derived Dickkopf1 is associated with bone metastasis and the mechanism involves the inhibition of osteoblast differentiation. Biochem Biophys Res Commun 2014;443:962–8. [DOI] [PubMed] [Google Scholar]
- [30].Zhuang X, Zhang H, Li X, et al. Differential effects on lung and bone metastasis of breast cancer by Wnt signalling inhibitor DKK1. Nat Cell Biol 2017;19:1274–85. [DOI] [PubMed] [Google Scholar]


