Figure 3.
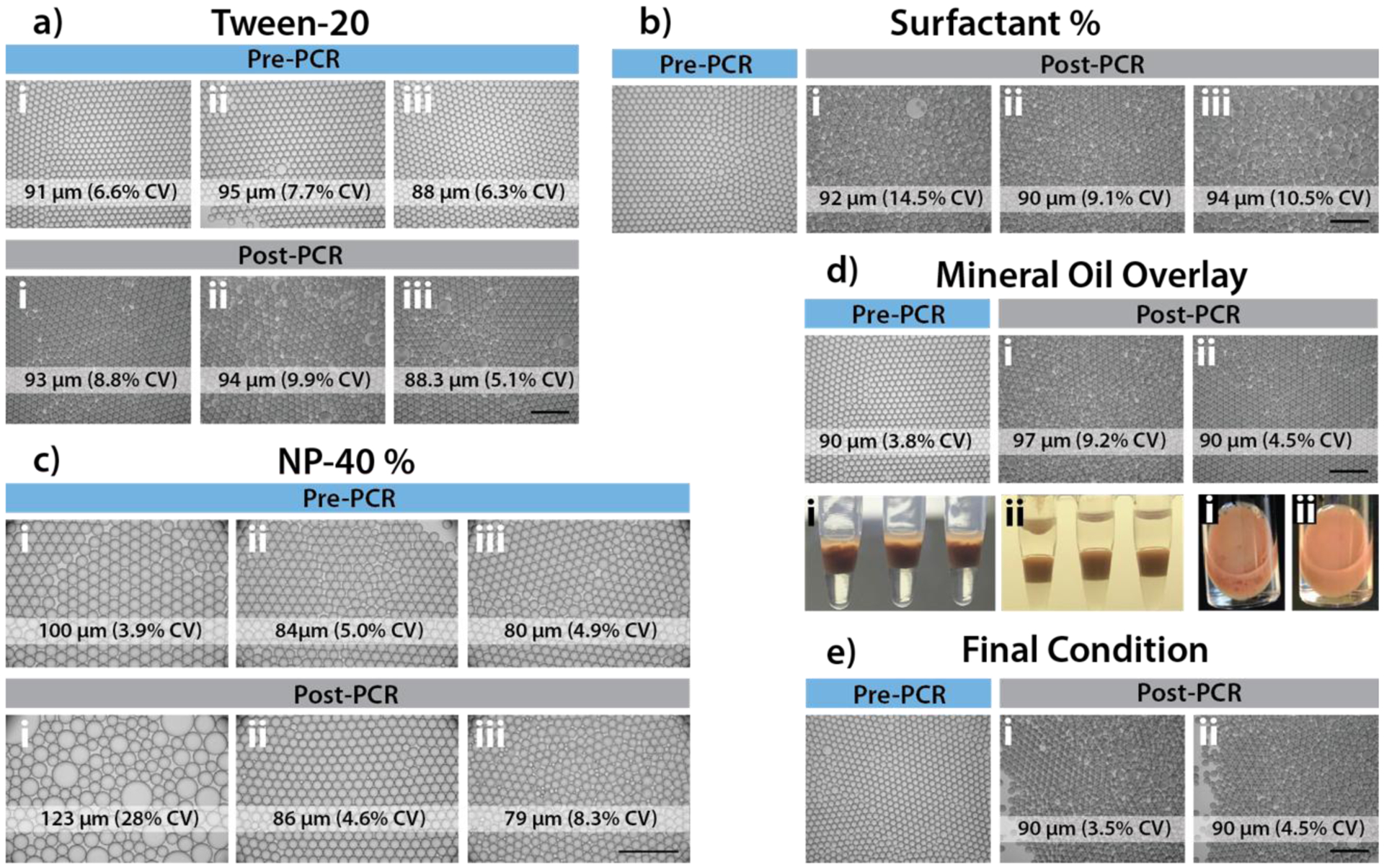
Blood droplet thermal stability optimization. a) Addition of Tween-20 to PCR mixture prior to encapsulation (i: 0%; ii: 0.01%; iii: 0.2%). Addition of Tween-20 was not found to significantly affect droplet thermal stability. b) Optimization of surfactant concentration for oil in PCR tubes. Emulsions of 2% surfactant with PCR mixtures containing 10% whole blood were loaded into PCR tubes containing preloaded various percentage of surfactant in HFE7500 (i: 2%; ii: 5%, iii: 10%). Droplets before and after PCR thermocycling were imaged (BF, 4x) and quantified for droplet CV. 5% surfactant oil in PCR tubes was found to be optimal. c) Optimization of NP-40 concentration in PCR buffer mixture prior to encapsulation (i: 0%; ii: 0.2%; iii: 0.4%). Final concentration of 0.2% NP-40 was found to result in improved droplet thermal stability. d) Addition of mineral oil overlay during 40 cycles of thermocycling (i: without mineral oil, ii: with 70 μl mineral oil overlay). Mineral oil overlay was found to significantly improve droplet thermal stability and yield. e) Final condition combining optimal parameters found in a-d. A portion of 10% whole blood droplets created with 2% PFPE surfactant oil were continuously mixed with 5% (w/w) surfactant in HFE7500 oil for 30 mins, before loading into PCR tubes. For PCR thermocycling, 70 μl of droplets (without mixing condition (i) or with mixing (ii)) were transferred into PCR tubes preloaded with 70 μl of mineral oil and 30 μl of 5% (w/w) surfactant in HFE7500 oil. The addition of a mixing step was not found to significantly improve droplet uniformity or thermal stability.
