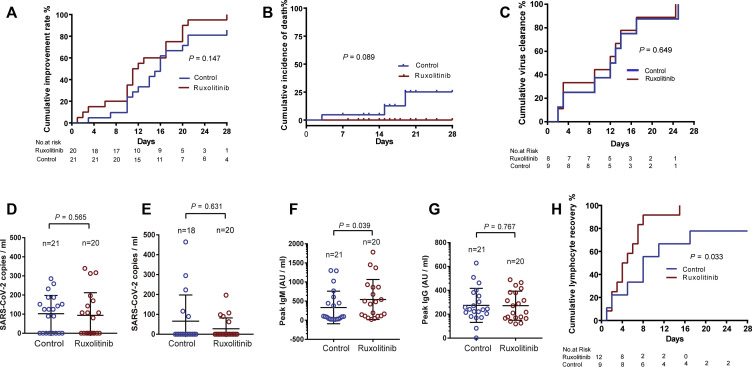
Fig 2.
Primary and secondary outcomes. A, The cumulative improvement rate in modified intention-to-treat analysis patients. B, Cumulative 28 days incidence of death. C, Cumulative incidence of virus clearance rate in analyzed patients. D, Comparison of blood viral loads of the control group and the ruxolitinib group at D1. E, Comparison of blood viral loads of the control group and the ruxolitinib group at discharge. F and G, The peak levels of SARS-CoV-2–specific IgM and IgG. H, Cumulative incidence of lymphocyte recovery rate in analyzed patients.