Abstract
To clarify the diversity of plant-parasitic Alternaria species in Japan, diseased samples were collected, and fungal isolates established in culture. We examined 85 isolates representing 23 species distributed in 14 known sections based on conidial morphology and DNA phylogeny. Three species were found to be new, A. cylindrica, A. paragomphrenae and A. triangularis. Furthermore, a lectotype was designated for A. gomphrenae, and epitypes for A. cinerariae, A. gomphrenae, A. iridicola, and A. japonica. Species boundaries of isolates were also clarified by studying phenotypes and determining host ranges. Alternaria gomphrenae and related species in sect. Alternantherae were recognized as distinct species owing to their host specificity. Among the species infecting Apiaceae, the pathogenicity of A. cumini and a novel species, A. triangularis ex Bupleurum, were confirmed as host specific. Another novel species, A. cylindrica, proved to be host specific to Petunia. Alternaria iridicola was recognized as a large-spored species in sect. Alternaria, being host specific to Iris spp. On the other hand, the experimental host ranges of three morphologically and phylogenetically distinct species infecting Brassicaceae (A. brassicae, A. brassicicola, and A. japonica) showed almost no differences. Alternaria brassicicola and A. porri were even found on non-host plants. In general, host ranges of Alternaria species correlated with morphology and molecular phylogeny, and combining these datasets resulted in clearer species boundaries.
Keywords: host range, morphology, new taxa, phenotyping, phylogeny, taxonomy
INTRODUCTION
Alternaria is a genus in the phylum Ascomycota (Pleosporaceae, Pleosporales) characterized by phaeodictyospores or phaeophragmospores (Seifert et al. 2011), and is one of the most ubiquitous fungal genera, inhabiting nearly every environmental substrate (atmosphere, soil, litter, and living plants) (Guo et al. 2004, Kirk et al. 2008). They are often allergenic, and can cause mycoses in humans and insects (Rossmann et al. 1996, Christias et al. 2001, Downs et al. 2001), but most species are plant pathogenic (Yu 2001). Alternaria species usually cause leaf spot diseases, especially on vegetables and ornamental flowers. However, it is their seed-borne phase that carries the greatest economic importance (Groves & Skolko 1944, Neergaard 1945, Richardson 1990, Tohyama 1993, Rathod 2012).
Alternaria was established and originally typified by Alternaria tenuis, and was redefined as a genus related to Stemphylium and Ulocladium based on its mode of conidiogenesis (Simmons 1967). Two additional genera, Embellisia and Nimbya, were subsequently established by Simmons (1971, 1989). The taxonomy of Alternaria and allied genera was previously based on conidial morphology, sporulation patterns, and differences in their host plants (mostly at the rank of genus) or substrates (Simmons 2007). However, their morphological variation and fundamental pleomorphism complicated species recognition, and thus host plants played a key role in identification. Due to their ubiquitous nature, this approach led to a false inflation of species numbers, resulting in the genus containing more than 400 species (Nishikawa & Nakashima 2015, Lawrence et al. 2016). The introduction of a molecular phylogenetic approach has again helped to clarify their taxonomy, reducing many allied genera into one large genus, Alternaria (Woudenberg et al. 2013).
Despite the application of molecular phylogenetic analyses, the relationship between taxonomy and plant parasitism remain insufficient to aid the practical recognition of species boundaries, and additional characterization is needed. Many of the phylogenetic species described by Woudenberg et al. (2013) were defined without morphological and pathological features able to distinguish closely related species. Therefore, we proposed an integrated species recognition based on morphology, molecular phylogeny, and pathogenicity (Nishikawa & Nakashima 2013). In our previous studies, it was suggested that phenotyping combined with a clarification of the host range via inoculation studies was helpful to resolve species boundaries (Nishikawa & Nakashima 2013, 2015).
During the survey of Japanese species of Alternaria, we collected and examined 85 isolates, and applied the integrated species recognition method to all Japanese species. The present study focused on biodiversity and the utility of phenotyping based on systematic experimental host range determination by inoculation tests. In addition, morphological observations and phylogenetic analyses were conducted to distinguish closely related species infecting Amaranthaceae, Apiaceae, Brassicaceae, Iridaceae, and Solanaceae.
MATERIALS AND METHODS
Fungal collection and isolation
The 85 isolates examined in the present study were obtained from diseased leaves, stems, buds, rhizomes, and seeds of various plants on the basis of field surveys in Japan from 2002 to 2018 (Table 1). Some of the isolates and specimens were obtained from the culture collection at the Genetic Resources Center of the National Agriculture and Food Research Organization (NARO; MAFF), Tsukuba, Japan, and several collaborators who assisted in the acquisition of these specimens are mentioned in the acknowledgements. To establish axenic cultures originating from single conidia, alternarioid conidia from lesions were suspended in sterilized distilled water and spread on 2 % water agar (WA) medium using a flame-sterilized microspatula. After incubation at 20 °C for 24 h, individual germinating conidia were transferred to potato-carrot agar (PCA; Simmons 2007) using a flame-sterilized microtube under a dissecting microscope at ×100 magnification (Nakashima et al. 2011). Deposits of the representative isolates from the present study were made in NARO and Mie University (MUCC), Tsu, Mie, Japan. Specimens, including holotype and epitype specimens were deposited in TNS (National Museum of Nature and Science), Tsukuba, Ibaraki, Japan, and/or in TSU (Mie University).
Table 1.
Isolates of Japanese species of Alternaria obtained in this study.
| Fungal name | Alternaria section | Strain number1,2 | Host plant | Location; year |
|---|---|---|---|---|
| Alternaria alstroemeriae | Alternaria | |||
| MAFF 241374 | Alstroemeria sp. | Nagano Pref., Matsumoto; 2008 | ||
| Alternaria alternata | Alternaria | |||
| MAFF 239887 | Vigna radiata | unknown (Japan); 1998 | ||
| MUCC 1610 | Impatiens hawkeri | Nagano Pref., Azumino; 2006 | ||
| MUCC 1611 | Antirrhinum majus | Shizuoka Pref., Kakegawa; 2008 | ||
| MUCC 1616 | Pelargonium hortorum | Kanagawa Pref. Nakai; 2004 | ||
| MUCC 1617 | Primula × polyantha | Shizuoka Pref., Kakegawa; 2004 | ||
| AC82 | Solanum lycopersicum | Shizuoka Pref., Kakegawa; 2011 | ||
| MAFF 243775 | Vigna radiata | Tokyo, Chiyoda; 2012 | ||
| MAFF 305014 | Pyrus aromatica | Kanagawa Pref.; 1958 | ||
| MAFF 410775 | Unknown (Pyrus?) | Unknown (Japan) | ||
| Alternaria atra | Ulocladioides | |||
| AC86 | Raphanus sativus | Tokyo, Setagaya; 2000 | ||
| AC87 | Brassica oleracea var. capitata | Tokyo, Setagaya; 2001 | ||
| AC88 | Brassica rapa subsp. pekinensis | Tokyo, Setagaya; 2001 | ||
| MAFF 246889 | Allium fistulosum | Tokyo, Setagaya; 2001 | ||
| Alternaria botrytis | Ulocladium | |||
| MAFF 246887 | Asparagus officinalis | Shizuoka Pref., Kakegawa; 2008 | ||
| Alternaria brassicae | ||||
| AC29 | Brassica rapa | Shizuoka Pref., Kakegawa; 2006 | ||
| MAFF 240791 | Raphanus sativus | Ibaraki Pref., Tsukuba; 2007 | ||
| MUCC 1615 | Raphanus sativus | Chiba Pref. Narita; 2009 | ||
| Alternaria brassicicola | Brassicicola | |||
| MAFF 246772 = MUCC 1694 | Brassica oleracea var. sabellica | Shizuoka Pref., Kakegawa; 2003 | ||
| MAFF 246773 | Spinacia oleracea | Tokyo, Setagaya; 2002 | ||
| MUCC 1612 = AC56 | Brassica rapa subsp. pekinensis | Shizuoka Pref., Kakegawa; 2008 | ||
| MUCC 1619 = AC70 | Raphanus sativus | Tokyo, Setagaya; 2000 | ||
| AC71 | Raphanus sativus | Tokyo, Setagaya; 2000 | ||
| AC72 | Brassica oleracea var. italica | Tokyo, Setagaya; 2001 | ||
| Alternaria celosiicola | Alternantherae | |||
| MAFF 243058 | Celosia argentea var. plumosa | Kanagawa Pref., Fujisawa; 2006 | ||
| Alternaria chartarum | Pseudoulocladium | |||
| MAFF 246888 | Capsicum annuum | Tokyo, Setagaya; 2000 | ||
| Alternaria cinerariae | Sonchi | |||
| MAFF 243059 = MUCC 1701ET | Pericallis cruenta | Chiba Pref., Narita; 2002 | ||
| MAFF 241266 = MUCC 1613 | Farfugium japonicum | Ibaraki Pref., Tsukuba; 2008 | ||
| MAFF 241267 = MUCC 1614 | Gynura bicolor | Ibaraki Pref., Tsukuba; 2008 | ||
| MUCC 2504 | Jacobaea maritima | Kanagawa Pref., Atsugi; 2017 | ||
| Alternaria crassa | Porri | |||
| MAFF 243056 | Datura stramonium | Tokyo, Kodaira; 2000 | ||
| MUCC 2502 = 12-M0180 | Datura fastuosa | Tokyo, Kodaira; 2012 | ||
| MUCC 2503 = 12-M0099 | Datura inoxia | Tokyo, Kodaira; 2012 | ||
| Alternaria cucumerina | Porri | |||
| AC105 | Cucurbita maxima | Niigata Pref., Sado; 2010 | ||
| AC106 | Cucurbita maxima | Niigata Pref., Sado; 2010 | ||
| Alternaria cumini | Eureka | |||
| MAFF 246774 | Cuminum cyminum | Shizuoka Pref., Kakegawa; 2012 | ||
| AC115 | Cuminum cyminum | Shizuoka Pref., Kakegawa; 2013 | ||
| Alternaria cyrindrica* | Alternaria | |||
| MAFF 246770T | Petunia × atkinsiana | Shizuoka Pref., Kakegawa; 2006 | ||
| Alternaria dauci | Porri | |||
| MUCC 1684 | Daucus carota | Shizuoka Pref., Kakegawa; 1998 | ||
| AC9 | Daucus carota | Shizuoka Pref., Kakegawa; 1998 | ||
| Alternaria gaisen f. sp. fragariae | Alternaria | |||
| MAFF 242310 = MUCC 1609 | Fragaria × ananassa ‘HS-138’ | Hokkaido, Esashi; 2007 | ||
| MAFF 731001 | Fragaria × ananassa ‘Morioka-16’ | Iwate Pref., Morioka; 1975 | ||
| MAFF 731002 | Fragaria × ananassa ‘Morioka-16’ | Iwate Pref., Morioka; 1975 | ||
| MAFF 731003 | Fragaria × ananassa ‘Morioka-16’ | Iwate Pref., Morioka; 1975 | ||
| MAFF 731004 | Fragaria × ananassa ‘Morioka-16’ | Iwate Pref., Morioka; 1975 | ||
| MAFF 731005 | Fragaria × ananassa ‘Morioka-16’ | Iwate Pref., Morioka; 1975 | ||
| MAFF 731006 | Fragaria × ananassa ‘Morioka-16’ | Iwate Pref., Morioka; 1975 | ||
| MAFF 731007 | Fragaria × ananassa ‘Morioka-16’ | Iwate Pref., Morioka; 1975 | ||
| Alternaria gaisen f. sp. pyri | Alternaria | |||
| MUCC 2151 = 9901A | Pyrus pyrifolia var. culta ‘Nijisseiki’ | Tottori Pref., Tohaku; 1999 | ||
| MUCC 2152 = 9903A | Pyrus pyrifolia var. culta ‘Nijisseiki’ | Tottori Pref., Tohaku; 1999 | ||
| MUCC 2153 = 9904C | Pyrus pyrifolia var. culta ‘Nijisseiki’ | Tottori Pref., Tohaku; 1999 | ||
| Alternaria gomphrenae | Alternantherae | |||
| MAFF 246769 = MUCC 1623ET | Gomphrena globosa | Shizuoka Pref., Kakegawa; 2011 | ||
| Alternaria iridicola | Alternaria | |||
| MUCC 2148 | Iris japonica | Tokyo, Kodaira; 2010 | ||
| MAFF 246890 = MUCC 2149ET | Iris japonica | Kanagawa Pref., Kamakura; 2013 | ||
| MAFF 246771 = MUCC 2501 | Iris japonica | Shizuoka Pref., Fukuroi; 2018 | ||
| Alternaria japonica | Japonicae | |||
| AC73 | Raphanus sativus | Tokyo, Setagaya; 2000 | ||
| MAFF 246775 = MUCC 1622ET | Raphanus sativus | Tokyo, Setagaya; 2000 | ||
| AC96 | Brassica oleracea var. italica | Shizuoka Pref., Kakegawa; 2010 | ||
| AC97 | Brassica oleracea var. italica | Shizuoka Pref., Kakegawa; 2010 | ||
| Alternaria nobilis | Gypsophilae | |||
| AC1 | Dianthus barbatus | Shizuoka Pref., Kakegawa; 2003 | ||
| AC25 | Dianthus caryophyllus | Miyagi Pref., Sendai; 2002 | ||
| Alternaria panax | Panax | |||
| MUCC 1692 = PFAlt1-1 | Polyscias fruticosa | Tokyo, Ogasawara (Bonin Is.); 2003 | ||
| AC19 = PGAlt1 | Polyscias guilfoylei | Tokyo, Ogasawara (Bonin Is.); 2003 | ||
| MAFF 243161 = MUCC 1625 | Polyscias fruticosa | Tokyo, Ogasawara (Bonin Is.); 2011 | ||
| MAFF 243162 = MUCC 1626 | Polyscias fruticosa | Tokyo, Ogasawara (Bonin Is.); 2011 | ||
| Alternaria paragomphrenae* | Alternantherae | |||
| MAFF 246768 = MUCC 1683T | Gomphrena haageana | Shizuoka Pref., Hamamatsu; 2004 | ||
| Alternaria penicillata | Crivellia | |||
| MUCC 1657 | Papaver nudicaule | Tokyo, Tachikawa; 2005 | ||
| Alternaria petroselini | Radicina | |||
| MAFF 243057 | Petroselinum crispum | Shizuoka Pref., Kakegawa; 2007 | ||
| Alternaria porri | Porri | |||
| AC2 | Viola × wittrockiana | Shizuoka Pref., Kakegawa; 2003 | ||
| AC6 | Calibrachoa sp. | Shizuoka Pref., Kakegawa; 2004 | ||
| MUCC 1688 | Allium fistulosum | Shizuoka Pref., Kakegawa; 2004 | ||
| AC15 | Allium fistulosum | Saitama Pref.; 2004 | ||
| AC16 | Allium fistulosum | Gunma Pref., Takasaki; 2005 | ||
| AC17 | Allium fistulosum | Gunma Pref., Takasaki; 2005 | ||
| MUCC 1698 | Allium fistulosum | Gunma Pref., Tomioka; 2006 | ||
| AC32 | Allium fistulosum | Chiba Pref., Mobara; 2006 | ||
| MUCC 1702 | Eustoma exaltatum subsp. russellianum | Shizuoka Pref., Kakegawa; 2007 | ||
| AC68 | Allium fistulosum | Tokyo, Setagaya; 2001 | ||
| Alternaria triangularis* | ||||
| MAFF 246776T | Bupleurum rotundifolium | Kochi Pref., Konan; 2004 | ||
| AC95 | Bupleurum rotundifolium | Shizuoka Pref., Kakegawa; 2004 | ||
| Alternaria zinniae | Porri | |||
| MUCC 1704 | Zinnia hybrida | Nagano Pref., Tomi; 2007 | ||
| AC107 | Zinnia hybrida | Nagano Pref., Azumino; 2010 | ||
| AC108 | Zinnia elegans | Shizuoka Pref., Kakegawa; 2011 | ||
| AC109 | Zinnia elegans | Nagano Pref., Azumino; 2011 | ||
| Alternaria sp. | ||||
| Alternaria | MAFF 305015 | Pyrus aromatica | Chiba Pref.; 1959 |
1 AC: Personal collection of JN; MAFF: Genetic Resources Center, National Agriculture and Food Research Organization, Tsukuba, Japan; MUCC (Japan): Culture Collection, Laboratory of Plant Pathology, Mie University, Tsu, Japan.
2 Ex-type and -epitype strain indicated with T and ET, respectively.
* Novel taxa proposed in the taxonomy section.
Morphological observation and culture characteristics
For microscopic observations of diagnostic morphology comparable to those of Simmons’s standard conditions (2007), sporulation was induced according to methods reported by Nishikawa & Nakashima (2013). After incubation of the isolates at 25 °C in the dark for 7 d on PCA and V8 juice agar (V8; Simmons 2007), the growing colonies were scratched with a flame-sterilized microspatula and the aerial mycelia were removed to observe sporulation. Treated colonies in unsealed Petri dishes were incubated for 12–24 h at 25 °C under blacklight blue fluorescent lamps to induce sporulation, and then the plates were transferred to 20 °C in the dark. Caespituli that formed on the medium 7 d after incubation were mounted with Shear’s mounting fluid [300 mL aqueous potassium acetate (2 %), 120 mL glycerin, and 180 mL ethanol (95 %)]. The morphology of 100 conidia and other structures, such as conidiophores and chlamydospores, were examined at ×400 magnification, and sporulation patterns were also observed under a compound microscope. Morphological descriptions were made for examined isolates based on both media; however small-spored species (mostly in sect. Alternaria) were based on PCA, and large-spored species (mostly in sect. Porri) were based on V8 according to their comparable descriptions in Simmons (2007).
Mycelial discs of 85 isolates were plated onto potato-dextrose agar (PDA; 200 g potato, 20 g dextrose, and 20 g agar in 1.0 L distilled water) plates. The diameter of each of five colonies was measured after incubation in the dark for 7 d at 25 °C, and the mean diameters for a species were calculated with 95 % confidence intervals. Culture characteristics were also rated using the charts of Rayner (1970).
To induce sexual reproduction in our collected species, we applied the rice straw agar (RSA) method reported by Tanaka & Harada (2003). Rice straws 4–5 cm long were soaked in distilled water in a glass vial, autoclaved, and then three pieces of each straw were placed on WA. Mycelial discs of each isolate were plated and pre-incubated at 20 °C in the dark for 2 wk. To induce the production of ascomata, the plates were transferred and incubated under blacklight blue fluorescent lamp irradiation for 3 mo.
DNA extraction and phylogenetic analyses
An UltraClean Microbial DNA isolation kit (MoBio Laboratories, Carlsbad) was used to conduct DNA extraction according with manufacturer’s instructions. PCR amplification and sequencing of the rDNA internal transcribed spacer (ITS) region, glyceraldehyde-3-phosphate dehydrogenase (gapdh), RNA polymerase second largest subunit (rpb2), translation elongation factor 1-alpha (tef1), actin (act), Alternaria major allergen (Alt a 1), and endopolygalacturonase (endoPG) genes were conducted at the Mie University Advanced Science Research Promotion Center, according to the procedure described in previous studies (Nishikawa & Nakashima 2013, 2015, 2019). All the newly determined sequences were deposited in the DNA Data Bank of Japan (DDBJ) (Table 2). Complementary strands of the sequences were assembled and concatenated in MEGA v. 7 (Kumar et al. 2016) and were aligned using MAFFT v. 7 (Katoh et al. 2017; http://mafft.cbrc.jp/alignment/server/index.html). Sequence alignments were deposited in TreeBASE under number S24554.
Table 2.
Isolates and their accession numbers for phylogenetic analyses.
| Alternaria section | Fungal name and isolate numbers1,2 | Country, host plant | DDBJ/GenBank/EMBL accession numbers3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| ITS | gapdh | tef1 | rpb2 | Alt a 1 | endoPG | act | |||
| Alternantherae | Alternaria alternantherae (= Nimbya alternantherae) | ||||||||
| EGS52.039 | Unknown, Alternanthera philoxeroides? | JN383496 | JN383477 | JQ672485 | – | JN383511 | – | JQ671717 | |
| CBS 124392; HSAUP2798 | China, Solanum melongena | KC584179 | KC584096 | KC584633 | KC584374 | KP123846 | – | – | |
| A. celosiicola (= A. cristata) | |||||||||
| MAFF 243058 | Japan, Celosia argentea var. plumosa | AB678217 | AB744033 | LC480205 | LC476781 | AB744029 | – | AB744036 | |
| EGS42.013T | USA, Celosia cristata | JN383497 | JN383478 | JQ672483 | JQ646495 | JN383512 | – | JQ671716 | |
| A. gomphrenae (= N. gomphrenae) | |||||||||
| MAFF 246769; MUCC 1623ET | Japan, Gomphrena globosa | LC440579 | LC481999 | LC480206 | LC476782 | – | – | LC481857 | |
| A. paragomphrenae* | |||||||||
| MAFF 246768; MUCC 1683T | Japan, Gomphrena haageana | – | LC482000 | LC480207 | LC476783 | LC481610 | – | LC481858 | |
| A. perpunctulata (= N. perpunctulata) | |||||||||
| CBS 115267; EGS51.130T | USA, Alternanthera philoxeroides | KC584210 | KC584129 | KC584676 | KC584418 | JQ905111 | – | JQ671718 | |
| Alternaria | A. alstroemeriae | ||||||||
| MAFF 241374 | Japan, Alstroemeria sp. | AB678214 | AB744034 | LC275050 | LC275231 | AB744031 | LC276240 | AB744038 | |
| CBS 118809; EGS52.068T | Australia, Alstroemeria sp. | KP124297 | KP124154 | KP125072 | KP124765 | – | KP123994 | – | |
| A. alternata | |||||||||
| MAFF 239887 | Unknown, Vigna radiata | LC440580 | LC482001 | LC480208 | LC476784 | LC481611 | LC480946 | LC481859 | |
| MUCC 1610 | Japan, Impatiens hawkeri | LC269968 | LC270135 | LC275052 | LC275233 | LC276230 | LC276242 | LC481860 | |
| MUCC 1611 | Japan, Antirrhinum majus | LC440581 | LC270134 | LC275051 | LC275232 | LC276229 | LC276241 | LC481861 | |
| MUCC 1616 | Japan, Pelargonium hortorum | LC269969 | LC270136 | LC275053 | LC275234 | LC276231 | LC276243 | LC481862 | |
| MUCC 1617 | Japan, Primula polyantha | LC440582 | LC482002 | – | LC476785 | LC481612 | LC480947 | LC481863 | |
| AC82 | Japan, Solanum lycopersicum | LC440583 | LC482003 | LC480209 | LC476786 | LC481613 | LC480948 | LC481864 | |
| MAFF 243775 | Japan, Vigna radiata | LC164855 | LC169124 | LC167147 | LC476787 | LC167084 | – | – | |
| MAFF 305014 | Japan, Pyrus aromatica | LC164847 | LC482004 | LC167153 | LC476788 | LC481614 | LC480949 | – | |
| MAFF 410775 | Japan, unknown (Pyrus?) | LC164846 | LC482005 | LC167155 | LC476789 | LC167089 | LC480950 | – | |
| CBS 916.96; EGS34.016ET | India, Arachis hypogaea | AF347031 | AY278808 | KC584634 | KC584375 | AY563301 | JQ811978 | JQ671702 | |
| CBS 918.96; EGS34.015 (= A. tenuissimaR) | UK, Dianthus chinensis | AF347032 | AY278809 | KC584693 | KC584435 | AY563302 | KP124026 | JQ671703 | |
| CBS 121348; EGS50.070 (= A. platycodonisT) | China, Platycodon grandiflorus | KP124367 | KP124219 | KP125144 | KP124836 | KP123915 | KP124070 | – | |
| CBS 101.26 (as A. iridis) | Unknown | – | JQ646313 | JQ672475 | JQ646482 | JQ646396 | – | JQ671694 | |
| A. alternata f. sp. citri pathotype rough lemon (= A. limoniasperaeT) | |||||||||
| CBS 102595; EGS45.100; BMP0316 | USA, Citrus jambhiri | FJ266476 | AY562411 | KC584666 | KC584408 | AY563306 | KP124029 | JQ671704 | |
| A. alternata f. sp. citri pathotype tangerine (= A. toxicogenicaT) | |||||||||
| CBS 102600; EGS39.181; ATCC 38963 | USA, Citrus reticulata | KP124331 | KP124186 | KP125107 | KP124799 | KP123880 | KP124033 | – | |
| A. alternata f. sp. mali (= A. maliT) | |||||||||
| CBS 106.24; EGS38.029; ATCC 13963 | USA, Malus sylvestris | KP124298 | KP124155 | KP125073 | KP124766 | KP123847 | AY295020 | – | |
| A. arborescens species complex | |||||||||
| CBS 102605; EGS39.128; BMP0308T (= A. alternata tomato pathotype) | USA, Solanum lycopersicum | AF347033 | AY278810 | KC584636 | KC584377 | AY563303 | AY295028 | JQ671705 | |
| CBS 119544; EGS43.072 (= A. cerealisT) | New Zealand, Avena sativa | KP124408 | JQ646321 | KP125186 | KP124878 | KP123955 | KP124112 | JQ671708 | |
| CBS 124283 | Russia, Oryza sp. | KP124416 | KP124267 | KP125194 | KP124885 | KP123963 | KP124120 | – | |
| CPC 25266 | Austria, Pyrus sp. | KP124418 | KP124269 | KP125196 | KP124887 | KP123965 | KP124122 | – | |
| A. betae-kenyensis | |||||||||
| CBS 118810; EGS49.159T | Kenya, Beta vulgaris var. cicla | KP124419 | KP124270 | KP125197 | KP124888 | KP123966 | KP124123 | – | |
| A. burnsii | |||||||||
| CBS 107.38; EGS06.185T | India, Cuminum cyminum | KP124420 | JQ646305 | KP125198 | KP124889 | KP123967 | KP124124 | JQ671685 | |
| A. cylindrica* | |||||||||
| MAFF 246770T | Japan, Petunia × atkinsiana | LC440584 | LC482006 | LC480211 | LC476791 | LC481616 | LC480951 | LC481867 | |
| A. eichhorniae | |||||||||
| CBS 489.92; ATCC 22255T | India, Eichhornia crassipes | KC146356 | KP124276 | KP125204 | KP124895 | KP123973 | KP124130 | – | |
| A. gaisen f. sp. fragariae (= A. alternata strawberry pathotype) | |||||||||
| MAFF 242310; MUCC 1609 | Japan, Fragaria × ananassa ‘HS-138’ | LC269973 | LC270141 | LC275059 | LC275239 | LC276237 | LC276252 | LC481865 | |
| MAFF 731001 | Japan, Fragaria × ananassa ‘Morioka-16’ | LC164854 | LC169125 | LC167148 | LC169131 | LC276235 | LC276246 | – | |
| MAFF 731002 | Japan, Fragaria × ananassa ‘Morioka-16’ | LC164853 | LC169126 | LC167149 | LC169132 | LC276236 | – | – | |
| MAFF 731003 | Japan, Fragaria × ananassa ‘Morioka-16’ | LC164852 | LC169127 | LC167150 | LC169133 | LC167085 | LC276247 | – | |
| MAFF 731004 | Japan, Fragaria × ananassa ‘Morioka-16’ | LC164851 | LC270140 | LC167151 | LC275238 | – | LC276248 | – | |
| MAFF 731005 | Japan, Fragaria × ananassa ‘Morioka-16’ | LC164850 | LC169128 | LC167152 | LC169134 | LC167086 | LC276249 | – | |
| MAFF 731006 | Japan, Fragaria × ananassa ‘Morioka-16’ | LC164849 | LC169129 | LC275057 | LC169135 | LC167087 | LC276250 | – | |
| MAFF 731007 | Japan, Fragaria × ananassa ‘Morioka-16’ | LC164848 | LC169130 | LC275058 | LC169136 | LC167088 | LC276251 | – | |
| A. gaisen f. sp. pyri (= A. alternata Japanese pear pathotype) | |||||||||
| CBS 118488; EGS90.0391ET | Japan, Pyrus pyrifolia var. culta ‘Nijisseiki’ | KP124427 | KP124278 | KP125206 | KP124897 | KP123975 | KP124132 | – | |
| CBS 632.93; EGS90.0512R | Japan, Pyrus pyrifolia var. culta ‘Nijisseiki’ | KC584197 | KC584116 | KC584658 | KC584399 | KP123974 | AY295033 | – | |
| MUCC 2151; 9901A | Japan, Pyrus pyrifolia var. culta ‘Nijisseiki’ | LC269970 | LC270137 | LC275054 | LC275235 | LC276232 | – | – | |
| MUCC 2152; 9903A | Japan, Pyrus pyrifolia var. culta ‘Nijisseiki’ | LC269971 | LC270138 | LC275055 | LC275236 | LC276233 | LC276244 | – | |
| MUCC 2153; 9904C | Japan, Pyrus pyrifolia var. culta ‘Nijisseiki’ | LC269972 | LC270139 | LC275056 | LC275237 | LC276234 | LC276245 | – | |
| A. gossypina | |||||||||
| CBS 104.32T | Zimbabwe, Gossypium sp. | KP124430 | JQ646312 | KP125209 | KP124900 | JQ646395 | KP124135 | JQ671693 | |
| A. iridiaustralis | |||||||||
| CBS 118486T; EGS43.014 | Australia, Iris sp. | KP124435 | KP124284 | KP125214 | KP124905 | KP123981 | KP124140 | – | |
| A. iridicola | |||||||||
| MUCC 2148 | Japan, Iris japonica | LC269974 | LC270142 | LC275060 | LC275240 | LC276238 | LC276253 | – | |
| MAFF 246890; MUCC 2149ET | Japan, Iris japonica | LC269975 | LC270143 | LC275061 | LC275241 | LC276239 | LC276254 | – | |
| MAFF 246771; MUCC 2501 | Japan, Iris japonica | – | – | LC480210 | LC476790 | LC481615 | – | LC481866 | |
| A. jacinthicola | |||||||||
| CBS 133751T | Mali, Eichhornia crassipes | KP124438 | KP124287 | KP125217 | KP124908 | KP123984 | KP124143 | – | |
| A. longipes (= A. alternata tobacco pathotype) | |||||||||
| CBS 540.94; EGS30.033R | USA, Nicotiana tabacum | AY278835 | AY278811 | KC584667 | KC584409 | AY563304 | KP124147 | JQ671689 | |
| CBS 121332; EGS30.048R | USA, Nicotiana tabacum | KP124443 | KP124292 | KP125222 | KP124913 | KP123989 | KP124149 | – | |
| A. tomato | |||||||||
| CBS 114.35 | Unknown, Solanum lycopersicum | KP124446 | KP124295 | KP125225 | KP124916 | KP123992 | KP124152 | JQ671686 | |
| Brassicicola | A. brassicicola | ||||||||
| MAFF 246772; MUCC 1694 | Japan, Brassica oleracea var. sabellica | LC440585 | LC482007 | LC480212 | LC476792 | LC481617 | – | LC481868 | |
| MAFF 246773 | Japan, Spinacia oleracea | – | LC482008 | LC480213 | LC476793 | LC481618 | – | LC481869 | |
| MUCC 1612; AC56 | Japan, Brassica rapa var. glabra | LC440586 | AB862969 | AB862981 | AB862975 | LC481619 | – | LC481870 | |
| MUCC 1619; AC70 | Japan, Raphanus sativus | LC440587 | AB862968 | AB862980 | AB862974 | LC481620 | – | LC481871 | |
| AC71 | Japan, Raphanus sativus | LC440588 | LC482009 | LC480214 | LC476794 | LC481621 | – | LC481872 | |
| CBS 118699; EGS42.002; ATCC 96836R | USA, Brassica oleracea | JX499031 | KC584103 | KC584642 | KC584383 | – | – | – | |
| A. conoidea (= Embellisia conoidea) | |||||||||
| CBS 132.89 | Saudi Arabia, Ricinus communis | FJ348226 | FJ348227 | KC584711 | KC584452 | FJ348228 | – | JQ671667 | |
| A. mimicula | |||||||||
| CBS 118696; EGS01.056; BMP0324T | USA, Solanum lycopersicum | FJ266477 | AY562415 | KC584669 | KC584411 | AY563310 | – | JQ671668 | |
| A. septorioides | |||||||||
| CBS 106.41; EGS52.089T | Netherlands, Reseda odorata | KC584216 | KC584136 | KC584685 | KC584427 | – | – | – | |
| A. solidaccana | |||||||||
| CBS 118698; EGS36.158T | Bangladesh, soil | KC584219 | KC584141 | KC584690 | KC584432 | – | – | – | |
| Chalastospora | A. cetera (= Chalastospora cetera) | ||||||||
| CBS 121340; CBS 110898; EGS41.072; BMP0033T | Australia, Elymus scabrus | JN383482 | AY562398 | KC584699 | KC584441 | AY563278 | – | JQ671626 | |
| Cheiranthus | A. cheiranthi | ||||||||
| CBS 109384; EGS41.188; BMP0148; BMP0148R | Italy, Cheiranthus cheiri | AF229457 | KC584107 | KC584646 | KC584387 | JQ905106 | – | JQ671656 | |
| Crivellia | A. papavericola (= Crivellia homothallica, Brachycladium papaveris) | ||||||||
| CBS 116606; P351T | USA, Papaver somniferum | FJ357310 | FJ357298 | KC584705 | KC584446 | JN383501 | – | JQ671608 | |
| A. penicillata (= Cr. papaveracea, B. penicillatum) | |||||||||
| MUCC 1657 | Japan, Papaver nudicaule | LC440589 | LC482010 | LC480215 | LC476795 | – | – | – | |
| CBS 116608; P354.8ET | Austria, Papaver rhoeas | FJ357311 | FJ357299 | KC584698 | KC584440 | JN383502 | – | JQ671609 | |
| CBS 116607; P354.1 | Austria, Papaver rhoeas | KC584229 | KC584153 | KC584706 | KC584447 | – | – | – | |
| Dianthicola | A. dianthicola | ||||||||
| CBS 116491; EGS51.022R | New Zealand, Dianthus × allwoodii | KC584194 | KC584113 | KC584653 | KC584394 | – | – | – | |
| Embellisia | A. embellisia (= E. allii) | ||||||||
| CBS 339.71R | USA, Allium sativum | KC584230 | KC584155 | KC584708 | KC584449 | – | – | – | |
| Embellisioides | A. hyacinthi (= E. hyacinthi) | ||||||||
| CBS 416.71; EGS19.102T | Netherlands, Hyacinthus orientalis | KC584233 | KC584158 | KC584716 | KC584457 | – | – | – | |
| Euphorbiicola | A. euphorbiicola | ||||||||
| CBS 119410; EGS41.029R | USA, Euphorbia pulcherrima | KJ718173 | KJ718018 | KJ718521 | KJ718346 | – | – | – | |
| Eureka | A. cumini | ||||||||
| MAFF 246774 | Japan, Cuminum cyminum | LC440590 | LC482011 | LC480216 | LC476796 | LC481622 | – | LC481873 | |
| AC115 | Japan, Cuminum cyminum | LC440591 | LC482012 | LC480217 | LC476797 | LC481623 | – | – | |
| CBS 121329; EGS04.1581T | India, Cuminum cyminum | KC584191 | KC584110 | KC584650 | KC584391 | – | – | – | |
| A. eureka (= E. eureka) | |||||||||
| CBS 193.86; EGS36.103T | Australia, Medicago rugosa | JN383490 | JN383471 | KC584715 | KC584456 | JN383507 | – | JQ671596 | |
| Gypsophilae | A. ellipsoidea | ||||||||
| CBS 119674; EGS49.104T | USA, Dianthus barbatus | KC584196 | KC584115 | KC584655 | KC584396 | – | – | – | |
| A. gypsophilae | |||||||||
| CBS 107.41; EGS07.025T | Unknown, Gypsophila elegans | KC584199 | KC584118 | KC584660 | KC584401 | KJ718688 | – | JQ671682 | |
| A. nobilis | |||||||||
| AC1 | Japan, Dianthus barbatus | LC440592 | LC482013 | LC480218 | LC476798 | LC481624 | LC480952 | LC481874 | |
| AC25 | Japan, Dianthus caryophyllus | LC440593 | LC482014 | LC480219 | LC476799 | – | – | – | |
| CBS 116490; EGS51.027R | New Zealand, Dianthus caryophyllus | KC584208 | KC584127 | KC584673 | KC584415 | JQ646385 | – | JQ671680 | |
| A. saponariae | |||||||||
| CBS 116492; EGS49.199R | USA, Saponaria officinalis | KC584215 | KC584135 | KC584683 | KC584425 | – | – | – | |
| A. vaccariicola | |||||||||
| CBS 118714; EGS46.003T | USA, Vaccaria hispanica | KC584224 | KC584147 | KC584697 | KC584439 | JQ646384 | – | JQ671679 | |
| Infectoriae | A. infectoria | ||||||||
| CBS 210.86; EGS27.193T | UK, Triticum aestivum | AF347034 | AY278793 | KC584662 | KC584404 | FJ266502 | – | JQ671629 | |
| Japonicae | A. japonica | ||||||||
| AC73 | Japan, Raphanus sativus | LC440594 | LC482015 | LC480220 | LC476800 | LC481625 | – | LC481875 | |
| MAFF 246775; MUCC 1622ET | Japan, Raphanus sativus | LC440595 | LC482016 | LC480221 | LC476801 | LC481626 | – | LC481876 | |
| AC96 | Japan, Brassica oleracea var. italica | LC440596 | LC482017 | LC480222 | LC476802 | LC481627 | – | LC481877 | |
| AC97 | Japan, Brassica oleracea var. italica | LC440597 | LC482018 | LC480223 | LC476803 | LC481628 | – | LC481878 | |
| CBS 118390; EGS50.099R | USA, Brassica chinensis | KC584201 | KC584121 | KC584663 | KC584405 | – | – | – | |
| A. nepalensis | |||||||||
| CBS 118700; EGS45.073T | Nepal, Brassica sp. | KC584207 | KC584126 | KC584672 | KC584414 | – | – | – | |
| Nimbya | A. scirpicola (= N. scirpicola) | ||||||||
| CBS 481.90; EGS19.042R | UK, Scirpus sp. | KC584237 | KC584163 | KC584728 | KC584469 | – | – | – | |
| Panax | A. avenicola | ||||||||
| CBS 121459; EGS50.185T | Norway, Avena sp. | KC584183 | KC584100 | KC584639 | KC584380 | – | – | – | |
| A. dendropanacis | |||||||||
| CNU 085031T | Korea, Dendropanax morbifer | HQ203210 | KF516506 | KP877992 | KP877985 | KF516492 | – | – | |
| CNU 085033 | Korea, Aralia elata | – | KF516507 | KP877993 | KP877986 | KF516493 | – | – | |
| A. eryngii | |||||||||
| CBS 121339; EGS41.005; BMP0336R | Unknown, Eryngium sp. | JQ693661 | AY562416 | KC584656 | KC584397 | AY563313 | - | JQ671670 | |
| A. panax | |||||||||
| MUCC 1692; PFAlt-1 | Japan, Polyscias fruticosa | – | LC482019 | LC480224 | LC476804 | – | – | – | |
| AC19; PGAlt1 | Japan, Polyscias guilfoylei | LC440598 | LC482020 | LC480225 | LC476805 | – | – | – | |
| MAFF 243161; MUCC 1625 | Japan, Polyscias fruticosa | LC440599 | AB862972 | AB862984 | AB862978 | – | – | – | |
| MAFF 243162; MUCC 1626 | Japan, Polyscias fruticosa | LC440600 | LC482021 | LC480226 | LC476806 | – | – | – | |
| CBS 482.81; EGS29.180R | USA, Aralia racemosa | KC584209 | KC584128 | KC584675 | KC584417 | JQ646382 | – | JQ671672 | |
| CBS 116532; EGS46.157R [as A. araliae in Deng et al. (2015)] | New Zealand, Meryta sinclairii | JF417549 | JF417630 | JX213321 | JF417657 | JX213285 | – | – | |
| CBS 116535; EGS48.124R | USA, Panax quinquefolius | JF417562 | JF417643 | JX213334 | JF417670 | JX213298 | – | – | |
| CNU 085019 | Korea, Panax ginseng | – | KF516502 | KP877988 | KP877981 | KF516488 | – | – | |
| CNU 101004 [as A araliae in Deng et al. (2015)] | Korea, Aralia continentalis | – | KF516501 | KP877987 | KP877980 | KF516487 | – | – | |
| A. photistica | |||||||||
| CBS 212.86; EGS35.172; BMP0041T | UK, Digitalis purpurea | KC584212 | KC584131 | KC584678 | KC584420 | AY563282 | – | JQ671632 | |
| Phragmosporae | A. phragmospora (= E. phragmospora) | ||||||||
| CBS 274.70; EGS27.098T | Netherlands, soil | JN383493 | JN383474 | KC584721 | KC584462 | JN383509 | – | JQ671623 | |
| Porri | Alternaría allii | ||||||||
| CBS 107.28; EGS48.084T | Puerto Rico, Allium cepa | KJ718100 | KJ717954 | KJ718449 | KJ718274 | KJ718620 | – | – | |
| CBS 116701; EGS33.134R | USA, Allium cepa var. viviparum | KJ718103 | KJ717957 | KJ718452 | KJ718277 | KJ718623 | – | – | |
| A. crassa | |||||||||
| MAFF 243056 | Japan, Datura stramonium | AB678215 | AB744032 | LC480227 | LC476807 | AB744028 | – | AB744035 | |
| MUCC 2502; 12-M0180 | Japan, Datura fastuosa | LC440601 | LC482022 | LC480228 | LC476808 | – | – | – | |
| MUCC 2503; 12-M0099 | Japan, Datura inoxia | – | LC482023 | LC480229 | LC476809 | – | – | – | |
| CBS 110.38ET | Cyprus, Datura stramonium | KJ718147 | KJ717997 | KJ718495 | KJ718320 | KJ718665 | – | – | |
| CBS 109160; EGS45.075; BMP0180 (= A. capsiciT) | Australia, Capsicum annuum | KJ718148 | AY562408 | KJ718496 | KJ718321 | AY563298 | – | JQ671747 | |
| A. cucumerina | |||||||||
| AC105 | Japan, Cucurbita maxima | LC440602 | LC482024 | LC480230 | LC476810 | – | – | – | |
| AC106 | Japan, Cucurbita maxima | LC440603 | LC482025 | LC480231 | LC476811 | – | – | – | |
| CBS 117225; EGS41.127R | USA, Cucumis melo | KJ718154 | KJ718001 | KJ718502 | KJ718327 | KJ718669 | – | – | |
| CBS 116114; EGS35.123 (= A. loofahaeT) | USA, Luffa acutangula | KJ718153 | KJ718000 | KJ718501 | KJ718326 | KJ718668 | – | – | |
| A. dauci | |||||||||
| MUCC 1684 | Japan, Daucus carota | LC440604 | LC482026 | – | LC476812 | – | – | – | |
| AC9 | Japan, Daucus carota | LC440605 | LC482027 | – | LC476813 | – | – | – | |
| CBS 111.38NT | Italy, Daucus carota | KJ718158 | KJ718005 | KJ718506 | KJ718331 | KJ718673 | – | – | |
| A. macrospora | |||||||||
| CBS 117228; EGS50.1901 | USA, Gossypium barbadense | KC584204 | KC584124 | KC584668 | KC584410 | KJ718702 | – | – | |
| A. porri | |||||||||
| AC2 | Japan, Viola x wittrockiana | LC440606 | LC482028 | LC480232 | LC476814 | – | – | – | |
| AC6 | Japan, Calibrachoa sp. | LC440607 | LC482029 | LC480233 | LC476815 | – | – | – | |
| MUCC 1688 | Japan, Allium fistulosum | LC440608 | LC482030 | LC480234 | – | – | – | – | |
| AC16 | Japan, Allium fistulosum | LC440609 | LC482031 | LC480235 | LC476816 | – | – | – | |
| AC17 | Japan, Allium fistulosum | – | LC482032 | LC480236 | LC476817 | – | – | – | |
| MUCC 1698 | Japan, Allium fistulosum | LC440610 | LC482033 | LC480237 | LC476818 | – | – | – | |
| AC32 | Japan, Allium fistulosum | LC440611 | LC482034 | LC480238 | LC476819 | – | – | – | |
| MUCC 1702 | Japan, Eustoma exaltatum subsp. russellianum | LC440612 | LC482035 | LC480239 | LC476820 | – | – | – | |
| AC68 | Japan, Allium fistulosum | LC440613 | LC482036 | LC480240 | LC476821 | – | – | – | |
| CBS 116699; EGS48.152ET | USA, Allium cepa | KJ718218 | KJ718053 | KJ718564 | KJ718391 | KJ718727 | – | – | |
| CBS 116698; EGS48.147R | USA, Allium cepa | DQ323700 | KC584132 | KC584679 | KC584421 | KJ718726 | – | – | |
| A. pseudorostrata | |||||||||
| CBS 119411; EGS42.060; BMP0174T | USA, Euphorbia pulcherrima | JN383483 | AY562406 | KC584680 | KC584422 | AY563295 | – | JQ671737 | |
| A. solarli | |||||||||
| CBS 109157; EGS44.098R | USA, Solanum tuberosum | KJ718238 | GQ180080 | _ | KJ718413 | KJ718746 | _ | _ | |
| A. tagetica | |||||||||
| CBS 479.81; EGS33.081R | UK, Tagetes erecta | KC584221 | KC584143 | KC584692 | KC584434 | KJ718761 | – | – | |
| A. zinniae | |||||||||
| MUCC 1704 | Japan, Zinnia hybrida | LC440614 | LC482037 | LC480241 | LC476822 | – | – | – | |
| AC107 | Japan, Zinnia hybrida | LC440615 | LC482038 | LC480242 | LC476823 | – | – | – | |
| AC 108 | Japan, Zinnia elegans | LC440616 | LC482039 | LC480243 | LC476824 | – | – | – | |
| AC 109 | Japan, Zinnia elegans | LC440617 | LC482040 | LC480244 | LC476825 | – | – | – | |
| CBS 117223; EGS44.035R | New Zealand, Zinnia elegans | KJ718270 | KJ718096 | KJ718616 | KJ718445 | KJ718777 | – | – | |
| Pseudoalternaria | A. rosae | ||||||||
| CBS 121341; EGS41.130T | New Zealand, Rosa rubiginosa | JQ693639 | JQ646279 | JQ672414 | – | JQ646370 | – | JQ671628 | |
| Pseudoulocladium | A. chartarum (= Ulocladium chartarum) | ||||||||
| MAFF 246888 | Japan, Capsicum annuum | LC440618 | LC482041 | LC480245 | LC476826 | LC481629 | – | LC481879 | |
| CBS 200.67; ATCC 18044; BMP0359ET | Canada, Populus sp. | AF229488 | KC584172 | KC584741 | KC584481 | AY563319 | – | JQ671654 | |
| A. aspera (= Ul. arborescens) | |||||||||
| CBS 115269; EGS44.109T | Japan, Pistacia vera | KC584242 | KC584166 | KC584734 | KC584474 | KF533899 | – | – | |
| A. concatenata (= Ul. capsici) | |||||||||
| CBS 120006; HSAUPIII000 35T | China, Capsicum annuum | KC584246 | AY762950 | KC584740 | KC584480 | – | – | – | |
| A. septospora (= Ul. septosporum) | |||||||||
| CBS 109.38 | Italy, wood pulp | FJ266489 | FJ266500 | KC584747 | KC584487 | – | – | – | |
| Radicina | A. petroselini | ||||||||
| MAFF 243057 | Japan, Petroselinum crispum | AB678216 | – | LC480246 | LC476827 | AB744030 | – | AB744037 | |
| CBS 112.41; EGS06.196T | unknown, Petroselinum sativum | KC584211 | KC584130 | KC584677 | KC584419 | – | – | – | |
| CBS 109383; EGS09.159; BMP0144R | USA, Petroselinum crispum | AF229454 | AY278799 | JQ672455 | JQ646474 | AY563288 | – | JQ671677 | |
| A. radicina | |||||||||
| CBS 245.67; EGS03.145; ATCC 6503NT | USA, Daucus carota | KC584213 | KC584133 | KC584681 | KC584423 | FN689405 | – | – | |
| A. seiini | |||||||||
| CBS 109382; EGS25.198T | Saudi Arabia, Petroselinum crispum | AF229455 | AY278800 | KC584684 | KC584426 | FJ266504 | – | JQ671676 | |
| A. smyrnii | |||||||||
| CBS 109380; EGS37.093; BMP0147R | UK, Smyrnium olusatrum | AF229456 | KC584138 | KC584687 | KC584429 | AY563289 | – | JQ671675 | |
| Soda | A. kulundii | ||||||||
| CBS 137525; M313T | Russia, soil | KJ443262 | KJ649618 | KJ443219 | KJ443176 | – | – | – | |
| Sonchi | A. cinerariae | ||||||||
| MAFF 243059; MUCC 1701ET | Japan, Pericallis cruenta | AB906673 | AB906670 | LC480247 | LC476828 | AB906671 | – | AB906672 | |
| MAFF 241266; MUCC 1613 | Japan, Farfugium japonicum | LC440619 | AB862970 | AB862982 | AB862976 | LC481630 | – | LC481880 | |
| MAFF 241267; MUCC 1614 | Japan, Gynura bicolor | LC440620 | AB862971 | AB862983 | AB862977 | LC481631 | – | LC481881 | |
| MUCC 2504 | Japan, Jacobaea maritima | LC440621 | LC482042 | – | LC476829 | LC481632 | – | LC481882 | |
| CBS 116495; EGS49.102R | USA, iigularia sp. | KC584190 | KC584109 | KC584648 | KC584389 | – | – | – | |
| A. sonchi | |||||||||
| CBS 119675; EGS43.131R | Canada, Sonchus asper | KC584220 | KC584142 | KC584691 | KC584433 | – | – | – | |
| Teretispora | A. leucanthemi (= Teretispora leucanthemi) | ||||||||
| CBS 421.65; ATCC 16028; EGS10.0591 | Netherlands, Chrysanthemum maximum | KC584240 | KC584164 | KC584732 | KC584472 | – | – | – | |
| Ulocladioides | A. atra (= Ul. atrum) | ||||||||
| AC86 | Japan, Raphanus sativus | LC440622 | LC482043 | LC480248 | LC476830 | LC481633 | – | LC481883 | |
| AC87 | Japan, Brassica oleracea var. capitata | – | LC482044 | LC480249 | LC476831 | LC481634 | – | LC481884 | |
| AC88 | Japan, Brassica rapa subsp. pekinensis | LC440623 | LC482045 | LC480250 | LC476832 | LC481635 | _ | LC481885 | |
| MAFF 246889 | Japan, Allium fistulosum | LC440624 | LC482046 | LC480251 | LC476833 | LC481636 | – | LC481886 | |
| CBS 195.67; ATCC 18040; BMP0355ET | USA, soil | AF229486 | KC584167 | KC584735 | KC584475 | AY563318 | _ | JQ671660 | |
| A. cucurbitae (= Ul. cucurbitae) | |||||||||
| CBS 483.81; EGS31.021; BMP0351R | New Zealand, Cucumis sativus | FJ266483 | AY562418 | KC584743 | KC584483 | AY563315 | – | JQ671663 | |
| A. multiformis (= Ul. multiforme) | |||||||||
| CBS 102060; EGS31.005T | Canada, soil | FJ266486 | KC584174 | KC584744 | KC584484 | FJ266512 | – | JQ671664 | |
| A. cantlous (= Ul. cantlous) | |||||||||
| CBS 123007; HSAUP0209T | China, Cucumis melo | KC584245 | KC584171 | KC584739 | KC584479 | EU684146 | – | – | |
| A. heterospora (= Ul. solani) | |||||||||
| CBS 123376; HSAUP 0521T | China, Solanum lycopersicum | KC584248 | KC584176 | KC584748 | KC584488 | EU855805 | – | – | |
| Ulocladium | A. alternariae (= Sinomyces alternariae) | ||||||||
| CBS 126989; EGS46.004 | USA, Daucus carota | AY376642 | AY376329 | KC584730 | KC584470 | – | – | – | |
| A. botrytis (= Ul. botrytis) | |||||||||
| MAFF 246887 | Japan, Asparagus officinalis | LC440625 | LC482047 | LC480252 | LC476834 | LC481637 | – | LC481887 | |
| CBS 197.67; ATCC 18042ET | USA, air | KC584243 | KC584168 | KC584736 | KC584476 | – | – | – | |
| A. oudemansii (= Ul. oudemansii) | |||||||||
| CBS 114.07; ATCC 18047; IMI 124940; MUCL 18563; QM 1744T | Unknown | FJ266488 | KC584175 | KC584746 | KC584486 | FJ266514 | – | – | |
| Undifilum | A. bornmuelleri (= Undifilum bornmuelleri) | ||||||||
| DAOM 231361 | Austria, Securigera varia | FJ357317 | FJ357305 | KC584751 | KC584491 | JN383516 | – | JQ671610 | |
| Monotypie lineage | A. argyranthemi | ||||||||
| CBS 116530; EGS44.033T | New Zealand, Argyranthemum sp. | KC584181 | KC584098 | KC584637 | KC584378 | – | – | – | |
| A. brassicae | |||||||||
| AC29 | Japan, Brassica rapa | LC440626 | AB862967 | AB862979 | AB862973 | LC481638 | – | LC481888 | |
| MAFF 240791 | Japan, Raphanus sativus | LC440627 | LC482048 | LC480253 | LC476835 | LC481639 | – | LC481889 | |
| MUCC 1615 | Japan, Raphanus sativus | LC440628 | LC482049 | LC480254 | LC476836 | LC481640 | _ | LC481890 | |
| CBS116528; EGS38.032R | USA, Brassica oleracea | KC584185 | KC584102 | KC584641 | KC584382 | _ | _ | _ | |
| A. dennisii (= E. dennisii) | |||||||||
| CBS 476.90; EGS30.121T | Isle of Man, Senecio jacobaea | JN383488 | JN383469 | KC584713 | KC584454 | JN383505 | – | – | |
| A. helianthiinficiens | |||||||||
| CBS 208.86; EGS36.184T | USA, Helianthus annuus | JX101649 | KC584120 | EU130548 | KC584403 | – | – | – | |
| A. peucedani | |||||||||
| CNU 111485T | Korea, Peucedanum japonicum | KF728231 | KF889361 | – | – | KF889363 | – | – | |
| A. soliaridae | |||||||||
| CBS 118387; EGS33.024T | USA, soil | KC584218 | KC584140 | KC584689 | KC584431 | _ | _ | _ | |
| A. thalictrigena | |||||||||
| CBS 121712; CPC 13410T | Germany, Thalictrum sp. | EU040211 | KC584144 | KC584694 | KC584436 | – | – | – | |
| A. thlaspis (= E. thlaspis) | |||||||||
| EGS45.0691 | UK, Thlaspis caerulescentis | JN383495 | JN383476 | – | – | JN383510 | – | JQ671607 | |
| A. triangularis* | |||||||||
| MAFF 246776T | Japan, Bupleurum rotundifolium | LC440629 | LC482050 | LC480255 | LC476837 | LC481641 | – | LC481891 | |
| AC95 | Japan, Bupleurum rotundifolium | LC440630 | LC482051 | LC480256 | LC476838 | LC481642 | – | LC481892 | |
| Out group | Paradendryphiella salina (= E. annulata) | ||||||||
| CBS 302.84T | North Sea, Cancer pagurus | JN383486 | JN383467 | KC584709 | KC584450 | – | – | JQ671591 | |
1 AC: Personal collection of JN; ATCC: American Type Culture Collection, Virginia, USA; BMP: Personal collection of Dr. B.M. Pryor, School of Plant Sciences, University of Arizona, Arizona, USA; CBS: Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands; CNU: Culture Collection of Chungnam National University, Daejeon, Korea; CPC: Personal collection of Dr. P.W. Crous, housed at CBS; DAOM: Canadian Collection of Fungal Cultures, Ottawa, Canada; EGS: Personal collection of Dr. E.G. Simmons; HSAUP: Department of Plant Pathology, Shandong Agricultural University, China; MAFF: Genetic Resources Center, National Agriculture and Food Research Organization, Tsukuba, Japan; MUCC (Japan): Culture Collection, Laboratory of Plant Pathology, Mie University, Tsu, Japan; P: Personal collection of Dr. P. Inderbitzin, Department of Plant Pathology, Cornell University, New York, USA.
2 Ex-type, -neotype, and -epitype strain indicated with T, NT, and ET; R: representative strain by Simmons (2007). Fungal names between parentheses refer to the former name or the name under the pathotype concept (Nishimura 1980).
3 Japanese isolates examined and accession numbers newly generated in this study are indicated in boldface.
* Novel taxa proposed in the taxonomy section.
To analyze the relationships between Japanese isolates and existing species, and to correctly classify them in Alternaria sections following Woudenberg et al. (2013), maximum parsimony (MP), maximum likelihood (ML), and Bayesian inference (BI) analyses were conducted using a combined dataset composed of 80 gapdh, rpb2, and tef1 sequences generated from our collected Japanese isolates and other sequences from GenBank (Table 2). Maximum parsimony analyses were performed in PAUP v. 4.0b10 (Swofford 2003) using heuristic searches, each of which consisted of 100 random sequence additions and a tree-bisection-reconnection (TBR) algorithm for branch swapping. All the characters were unordered and unweighted, with alignment gaps treated as missing data. Clade robustness of the obtained trees was assessed using 1 000 bootstrap (BS) replications (Felsenstein 1985). Tree scores, including tree length (TL), consistency index (CI), retention index (RI), rescaled consistency index (RC), and homoplasy index (HI), were calculated. Maximum likelihood analyses were performed in RAxML-NG v. 0.6.0 BETA (Kozlov et al. 2018) using the GTR+FO+G model as the nucleotide substitution model and 100 BS replicates. Bayesian inference analyses were performed in BEAST v. 2.5.1 (Bouckaert et al. 2014). A nucleotide substitution model TN93 was selected by Kakusan4 software (Tanabe 2011). To estimate the posterior probabilities (PPs) of tree topologies, Metropolis-Coupled Markov Chain Monte Carlo searches (MCMCMC) were run for 30 M generations with trees sampled and saved every 1 000 generations until the average standard deviation of split frequencies reached 0.01 (stop value), which generated 18 001 trees from which the initial 12 000 trees were discarded as burn-in based on the effective sample size (ESS) calculated by Tracer v. 1.7.1 software package (Rambaut et al. 2018). After discarding, PPs were determined from the remaining trees. Sequences of Paradendryphiella salina (= E. annulata) (CBS 302.84) were used as the outgroup.
To evaluate the validity of ITS as the fungal barcoding gene for Alternaria, and to find phylogenetic species boundaries via multi-locus phylogeny, MP, ML, and BI analyses were conducted separately with the ITS dataset, which was composed of 74 sequences generated from our collected Japanese isolates and other sequences from GenBank (Table 2). Maximum parsimony analyses were performed in PAUP v. 4.0b10, with the same procedure and settings. Maximum likelihood analyses were performed in RAxML v. 8.1.17 (Stamatakis 2014), using the GTR+GAMMA model as the nucleotide substitution model and 100 BS replicates. Bayesian inference analyses were performed in BEAST v. 2.5.1, with the HKY+GAMMA model selected by Kakusan4. To estimate the PPs of tree topologies, MCMCMC were run for 20 M generations with trees sampled and saved every 1 000 generations. After discarding the initial 10 000 trees as burn-in, PPs were determined from the remaining trees. Sequences of P. salina were used as the outgroup.
To analyze the detailed relationships between Japanese isolates within sect. Alternaria, MP, ML, and BI analyses were conducted using a combined dataset of act, Alt a 1, endoPG, gapdh, rpb2, and tef1 sequences, which was composed of nine sequences generated from our collected Japanese isolates and other sequences from GenBank (Table 2). Maximum parsimony and ML analyses were performed in PAUP v. 4.0b10 and RAxML v. 8.1.17, respectively, using the same procedure and settings used for ITS analyses. Bayesian inference analyses were performed in BEAST v. 2.5.1 with the GTR+GAMMA model selected by Kakusan4. To estimate the PPs of tree topologies, MCMCMC were run for 10 M generations with trees sampled and saved every 1 000 generations, which generated 9 001 trees from which the initial 1 000 trees were discarded as burn-in. After discarding, PPs were determined from the remaining trees. Sequences of the Japanese isolate of A. nobilis (AC1) were also used as the outgroup. The generated trees were printed with FigTree v. 1.4.2 (Institute of Evolutionary Biology, University of Edinburgh, http://tree.bio.ed.ac.uk/software/figtree).
Inoculation tests
To determine the experimental host range of the obtained isolates, conidia produced on V8 medium as described above were washed with sterile distilled water containing 0.02 % polyoxyethylene (20) sorbitan monolaurate (Wako Pure Chemicals, Osaka), and used as inocula (Nishikawa & Nakashima 2013). The concentration of each conidial suspension was adjusted using a hemocytometer, and then each inoculum was sprayed onto mature leaves of potted plants (at least three replicates) until run-off. Closely related plant species from the same family as the original host source of each Alternaria species were also inoculated. Furthermore, the unrelated plant species ecorded as hosts were also inoculated to confirm potential host species, and to define host range boundaries of Alternaria pecies. Control plants were prepared and sprayed with sterile distilled water. All the inoculated plants were maintained in an incubator under moist conditions at 20 °C.
Virulent phenotypes were evaluated 7 d post-inoculation (dpi) using the index described by Chaerani et al. (2007) (0: no visible leaf lesions; 1: up to 10 % of leaf area affected; 2: 11–25 % of leaf area affected; 3: 26–50 % of leaf area affected; 4: 51–75 % of leaf area affected; and 5: more than 75 % of leaf area affected or the leaf abscised), and the means for each inoculated plant species were calculated as disease severity with 95 % confidence intervals. Given the importance of epidemiology to the obtained results, we also focused on whether sporulation was present or absent on the host lesions. Consequently, pathogenicity of examined isolates was determined by the disease severity, symptom, and sporulation on lesions to evaluate host ranges more accurately. Inoculated plants showing no symptoms within 7 dpi were observed continuously until 30 dpi.
Among sect. Alternantherae, two species on Gomphrena, namely A. gomphrenae and MAFF 246768 (A. paragomphrenae), were examined to determine host preference within Amaranthaceae, and differential plants for both species compared with those of allied taxa in this section. Three species on Brassicaceae, which were known to be polyphyletic and morphologically distinguishable from each other (Simmons 2007, Woudenberg et al. 2013), were inoculated onto 13 species of Brassicaceae (with two varieties of Brassica oleracea and four subspecies of B. rapa) to compare their host range. Moreover, previously recorded non-Brassicaceae host plants, e.g. Cucumis and Beta for A. brassicae (Simmons 2007), including that of closely related species, namely Solanum for A. mimicula, were also used to verify their validity as true hosts. Alternaria cumini and MAFF 246776 (A. triangularis) were inoculated onto 13 host species of Apiaceae, including Bupleurum, comparing the host range of the other pathogenic species on this family. MAFF 246770 (A. cylindrica) was inoculated onto Petunia and six species of Solanaceae to determine its host range compared to those of A. crassa and A. solani. Vigna and Zea, which are recorded as host species of Prathoda longissima (= A. longissima) (Deighton et al. 1698), were also used to examine conspecificity with the previously reported pathogen identified as A. longissima in Japan (Takano 2005). Two isolates of A. iridicola were examined to reveal their host range within Iridaceae, including Gladiolus and Iris ensata, which are additional natural hosts recorded in Korea (Yu 2001), and one of the original host species of A. iridiaustralis recorded in China (Luo et al. 2018), respectively. Two non-Iridaceae species were additionally inoculated because one of these, Allium, was regarded as susceptible by Elliot (1917). In addition, A. porri-like large-spored isolates obtained from Calibrachoa (AC6), Eustoma (MUCC 1702), and Viola (AC2) were inoculated on each original host and related plant species as well as Allium to identify these miscellaneous isolates. Koch’s postulates were also tested for the three novel species described in this study.
RESULTS
Molecular phylogeny
The combined alignment of the gapdh, rpb2, and tef1 datasets contained 189 sequences with a total of 1 567 characters. PCR amplification and sequencing from six Japanese isolates, e.g. the gapdh sequence of A. petroselini MAFF 243057, were unsuccessful. The topologies of the resulting trees from MP, ML, and BI analyses were congruent, and Fig. 1 shows the ML tree with BS values (MP and ML) and Bayesian PP. The Japanese Alternaria isolates examined were divided into 14 sections and two monotypic lineages as strongly supported clades; The Japanese isolate of A. petroselini clustered with species of sect. Radicina based on ITS, tef1, rpb2, Alt a 1, and act, respectively (data not shown). Five Japanese species clustered respectively in sect. Alternaria and Porri, and three species in sect. Alternantherae.
Fig. 1.
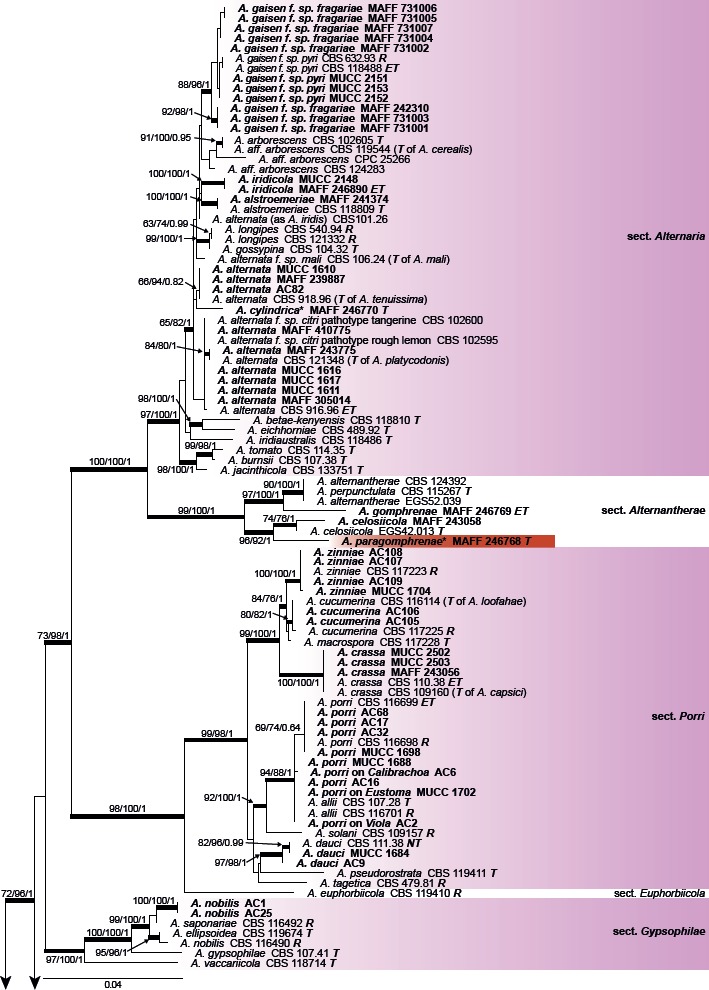
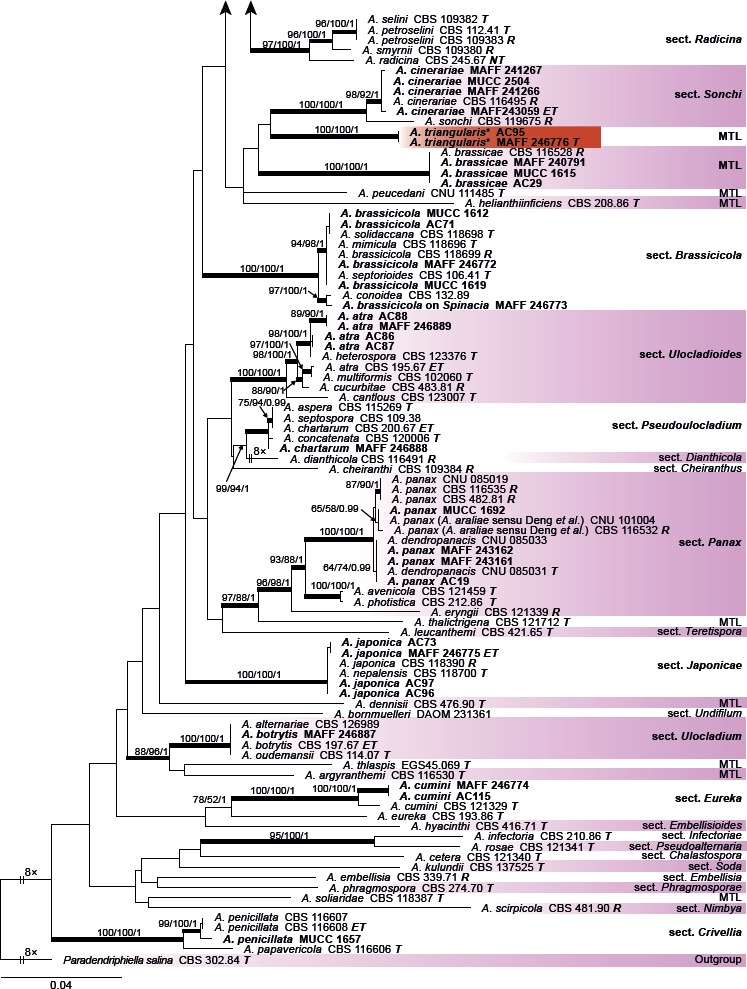
Maximum likelihood (ML) tree based on the combined dataset of gapdh, rpb2, and tef1 sequences from Japanese Alternaria isolates. The tree was rooted to Paradendryphiella salina (CBS 302.84). Maximum parsimony (MP) and ML bootstrap values and Bayesian posterior probabilities (PP) are given near branches (MP/ML/PP). Thickened nodes indicate significant support by MP/ML/PP (> 70/70/0.95). The scale bar indicates the number of nucleotide substitutions per site. Japanese isolates examined are indicated in bold, and the statuses of reference isolates are indicated in bold and italic. T: ex-type, NT: ex-neotype, ET: ex-epitype, R: representative strain assigned by Simmons (2007). Names of sections and monotypic lineages (MTL) for each taxon are given in the right column, and the Japanese isolates examined in the study are also indicated in bold. Resolved novel taxa with an asterisk were indicated as red shadings.
Among the three novel species identified based on their distinct morphological characteristics, MAFF 246768 ex G. haageana (A. paragomphrenae) was clearly distinguishable from A. gomphrenae and A. celosiicola in sect. Alternantherae, and two isolates (MAFF 246776 and AC95) ex Bupleurum (A. triangularis) were also well-resolved as a new monotypic sister lineage to sect. Sonchi. However, MAFF 246770 ex Petunia (A. cylindrica) had a unique sequence with strong BS support in ML but with weak support in MP and BI. The remaining other morphologically distinguishable species were assigned to each valid clade, whereas Japanese isolates of A. botrytis, A. brassicicola, A. chartarum, and A. japonica were indistinguishable from closely related taxa, including the ex-type and ex-epitype isolates in each section.
The ITS datasets containing 178 sequences were aligned for a total of 586 characters. The topologies of the resulting trees from MP, ML, and BI analyses were congruent, and Fig. 2 shows one of the MP trees (TL = 600, CI = 0.433, RI = 0.881, RC = 0.382, HI = 0.567) with BS values (MP and ML), and Bayesian PP. Almost all of the examined Japanese species, together with their closely related taxa in phylogenetic trees that were indistinguishable based on the combined gapdh, rpb2, and tef1 sequence datasets, were each recognized as separate species. Two isolates (MAFF 246776 and AC95) ex Bupleurum (A. triangularis) were well-resolved as a distinct new species. However, species in sect. Alternaria, and two large-spored species in sect. Porri having colored filamentous beaks (A. cucumerina and A. zinniae), were not recognized as independent species. PCR amplification and sequencing of seven Japanese isolates, i.e. MAFF 246768 (A. paragomphrenae) and A. iridicola MAFF 246771, were unsuccessful.
Fig. 2.
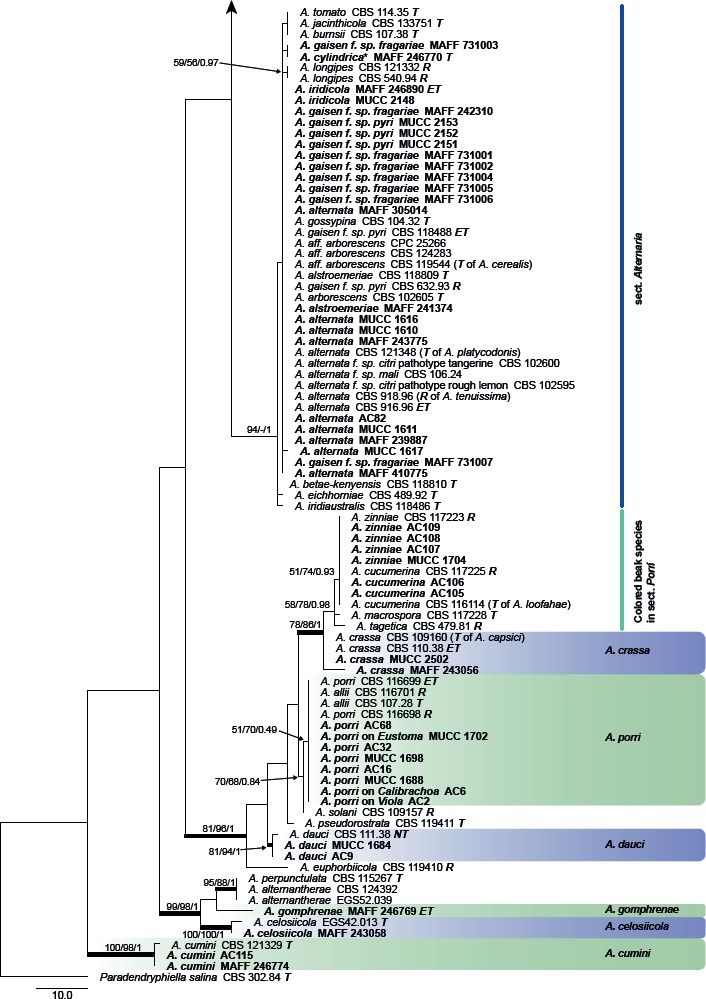
Phylogenetic tree generated from maximum parsimony (MP) analysis based on the ITS sequences from Japanese Alternaria isolates. The tree was rooted to Paradendryphiella salina (CBS 302.84). MP and RAxML maximum likelihood (ML) bootstrap values and Bayesian posterior probabilities (PP) are given near branches (MP/ML/PP). Thickened nodes indicate significant support by MP/ML/PP (> 60/60/0.96). Tree length = 600, consistency index = 0.433, homoplasy index = 0.567, retention index = 0.881, and rescaled consistency index = 0.382. The scale bar indicates the number of nucleotide substitutions. Japanese Alternaria isolates examined are indicated in bold, and the statuses of reference isolates are indicated in bold and italic. T: ex-type, NT: ex-neotype, ET: ex-epitype, R: representative strain assigned by Simmons (2007). Asterisks indicate novel taxa proposed in the taxonomy section.
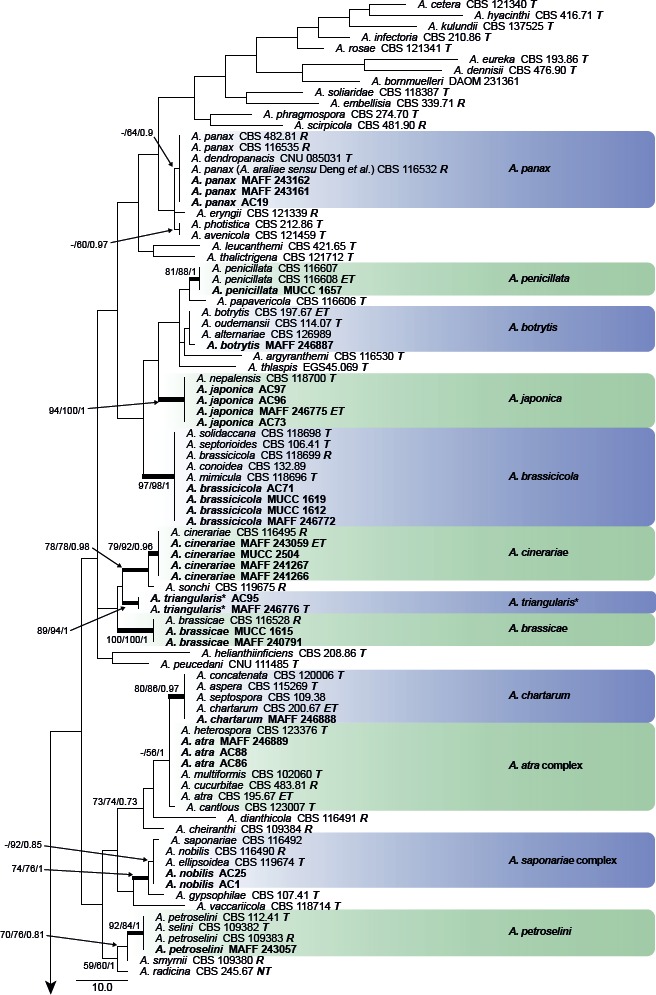
The combined alignment of act, Alt a 1, endoPG, gapdh, rpb2, and tef1 datasets contained 18 sequences with a total of 2 473 characters. PCR amplification and sequencing of the act sequence of A. iridicola MAFF 246890 was unsuccessful. The topologies of the resulting trees from MP, ML, and BI analyses were congruent, and Fig. 3 shows one of the MP trees (TL = 383, CI = 0.859, RI = 0.783, RC = 0.673, HI = 0.141) with BS values (MP and ML) and Bayesian PP. MAFF 246770 ex Petunia (A. cylindrica) was identified as a new sister lineage to the A. arborescens species complex in this section.
Fig. 3.
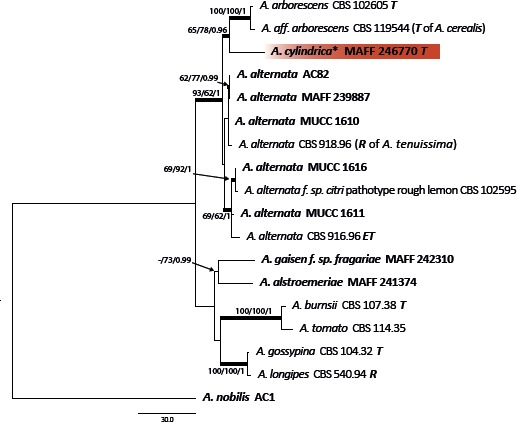
Phylogenetic tree of sect. Alternaria generated from maximum parsimony (MP) analysis based on the combined dataset of act, Alt a 1, endoPG, gapdh, rpb2, and tef1 sequences from 17 isolates. The tree was rooted to Alternaria nobilis (sect. Gypsophilae). MP and RAxML maximum likelihood (ML) bootstrap values and Bayesian posterior probabilities (PP) are given near branches (MP/ML/PP). Thickened nodes indicate significant support by MP/ML/PP (> 60/60/0.96). Tree length = 383, consistency index = 0.859, homoplasy index = 0.141, retention index = 0.783, and rescaled consistency index = 0.673. The scale bar indicates the number of nucleotide substitutions. Japanese isolates examined are indicated in bold, and statuses of reference isolates are indicated in bold and italic. T: ex-type, ET: ex-epitype, R: representative strain assigned by Simmons (2007). Resolved novel taxon with asterisk was indicated as red shadings.
Morphology and growth rate on potato-dextrose agar
Based on their conidial morphology on PCA and V8 media, the Japanese Alternaria isolates examined in the present study were recognized as either one of 23 existing species, or one of three novel species. One of the novel taxa, MAFF 246768 (A. paragomphrenae) ex Gomphrena haageana, produced very similar conidia to those of A. gomphrenae and other species in sect. Alternantherae; however, they differed in the length and width of their conidial bodies (Table 3). Among their various features, conidiophore width was a defining characteristic of each Alternaria section: those of sect. Alternaria [A. alstroemeriae, A. alternata, A. gaisen, and MAFF 246770 (A. cylindrica)], Brassicicola, Crivellia, Japonica, Pseudoulocladium, Ulocladioides, Ulocladium, and MAFF 246776 (A. triangularis) for the most part did not exceed an average of 5 µm (narrow conidiophores); those of sect. Alternantherae [A. celosiicola, A. gomphrenae, and MAFF 246768 (A. paragomphrenae)], Eureka, Gypsophilae, Panax, Porri (A. crassa, A. cucumerina, A. dauci, A. porri, A. zinniae), Sonchi, and A. brassicae usually reached 6–7 µm (thick conidiophores); those of sect. Radicina were of an intermediate width, ranging around 5–6 µm; and A. iridicola produced mostly narrow, but often thickened, conidiophores. In addition, species in sect. Porri were characterized by the morphology of their beaks, especially in color. Those of A. porri and A. dauci were typically hyaline, whereas those of A. cucumerina and A. zinniae were always colored. Moreover, A. crassa grown on V8 medium commonly also formed colored beaks as cylindrical secondary conidiophores, but not filamentous true beaks. A detailed morphology of each Japanese species examined in the present study follows in the taxonomy section.
Table 3.
Morphological comparisons among the species of sect. Alternantherae.
| Fungal species and isolates1 | Original host plants | Conidial bodies | Beaks | Substrates | References | ||||
|---|---|---|---|---|---|---|---|---|---|
| Length × width (μm) | Average with 95 % Cl | Transverse septa | Longitudinal septa | Length × width (μm) | Average with 95 % Cl | ||||
| Examined species | |||||||||
| Alternaría celosiicola | Celosía | ||||||||
| MAFF 243058 | 36–161 × 8–26 | 107±7 × 20±1 | 2–16 | 0–4 | 55–670 × 2–3 | 332±44 × 2±0.1 | PCA | Nishikawa & Nakashima (2013) | |
| 42–180 × 10–26 | 116±7 × 18±1 | 2–17 | 0–5 | 49–575 × 2–4 | 195±38 × 2±0.1 | V8 | Nishikawa & Nakashima (2013) | ||
| 68–173 × 13–26 | 119±5 × 20±1 | 8–15 | 0(–2) | 120–285 × 2–3 | 197±13 × 2±0.1 | Lesion | Nishikawa & Nakashima (2013) | ||
| EGS42.013T | 50–190 × 7–17 | – | 11–14 | 1–3 | 250–470 × 2–4 | – | PCA | Simmons (1995b) | |
| A. gomphrenae | Gomphrena | ||||||||
| MAFF 246769ET | 46–103 × 11–21 | 82±4 × 16±1 | 4–10 | 0(–1) | 18–188 × 2–4 | 87±10 × 3±0.1 | PCA | This study | |
| 35–77 × 10–17 | 58±2 × 14±0.4 | 3–9 | 0–1 | 13–216 × 2–4 | 97±14 × 3±0.1 | V8 | This study | ||
| 30–106 × 8–23 | 67±5 × 12±1 | 0–13 | 0 | 11–163 × 2–5 | 72±9 × 2±0.2 | Lesion | This study | ||
| – | 46–94 × 10–16 | 74±3 × 14±0.4 | 4–10 | 0 | – | – | Lesion | This study, lectotype | |
| – | 100 × 15 | – | 5–14 | – | up to 150 | – | Lesion | Togashi (1926); Simmons (1989) | |
| EGS40.146 | 80–100 × 18–20 | – | – | – | 60–80 × 2 | – | PCA | Simmons (1995b) | |
| – | 48–105 × 9–18 | 80 × 14 | – | rare | 33–111 × 2–3 | 69 × 3 | Lesion | Yoshii (1933) | |
| A. paragomphrenae* | Gomphrena | ||||||||
| MAFF 246768T | 60–111 × 15–25 | 87±3 × 20±1 | 2–9 | 0–3 | 14–208 × 2–5 | 129±11 × 3±0.1 | PCA | This study | |
| 48–98 × 17–33 | 76±3 × 25±1 | 3–7 | 0–4 | 25–316 × 3–5 | 150±18 × 3±0.2 | V8 | This study | ||
| 25–99 × 8–26 | 56±5 × 19±1 | 1–9 | 0–2 | 14–87 × 3–4 | 55±4 × 3±0.1 | Lesion | This study | ||
| Comparable species | |||||||||
| A. alternantherae | Alternanthera | ||||||||
| EGS39.124NT | 50–115 × 8–20 | – | 6–10 | 0–2 | 350–470 × 2–4 | – | PCA | Simmons (1995b) | |
| A. crassoides | Froelichia | ||||||||
| – | 45–90 × 8–20 | 7–9 | – | 40–60 × 2 | – | Lesion | Simmons (1995b), lectotype | ||
| A. perpunctulata | Alternanthera | ||||||||
| CBS 115267T | 80–100 × 10–14 | 10–15 | 1–2 | 100–210 | – | PCA | Simmons (2004) | ||
1 CBS: Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands; EGS: Personal collection of Dr. E.G. Simmons; MAFF: Genetic Resources Center, National Agriculture and Food Research Organization, Tsukuba, Japan. Ex-type, -neotype, and -epitype strain indicated with T, NT, and ET.
* Novel taxa proposed in the taxonomy section.
Colony diameters of the examined species ranged from 24–87 mm after 7 d incubation at 25 °C, and the mean with 95 % confidence intervals was 61.6 ± 6.3 mm (Fig. 4). Based on the mean colony diameters, the examined species were classified into groups; fast-growing: A. alstroemeriae, A. alternata, A. celosiicola, A. crassa, A. cucumerina, A. gaisen, A. petroselini, and A. porri; moderate-growing: A. atra, A. botrytis, A. brassicicola, A. chartarum, A. cinerariae, A. cumini, A. iridicola, A. japonica, A. zinniae, MAFF 246768 ex Gomphrena (A. paragomphrenae), and MAFF 246770 ex Petunia (A. cylindrica); slow to moderate-growing: A. dauci, A. gomphrenae, and A. panax; slow-growing: A. brassicae, A. nobilis, A. penicillata, and MAFF 246776 ex Bupleurum (A. triangularis).
Fig. 4.
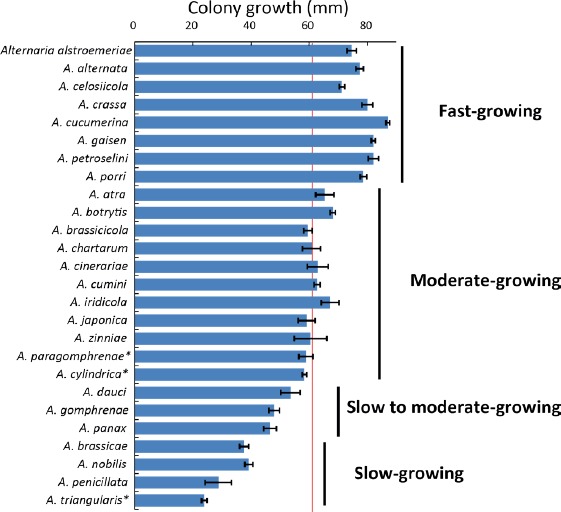
Mean colony diameters (mm) on potato-dextrose agar medium. The mean for the entire examined species is indicated as a red line at 61.6 mm. Bars indicate the 95 % confidence intervals. Asterisks indicate novel taxa proposed in the taxonomy section.
Experimental host range
Inoculation tests conducted in this study determined host ranges of each species, as well as species boundaries between closely related species. Moreover, “false” hosts, which were previously recorded as if true hosts or susceptible host plants, were also revealed. Detailed results are described for each host family as follows.
Alternaria gomphrenae and a novel species infecting Amaranthaceae
Two Alternaria isolates, A. gomphrenae MAFF 246769 and MAFF 246768 ex G. haageana (A. paragomphrenae), were inoculated onto Amaranthaceae plants by spraying a conidial suspension concentrated at an average of 2.2 × 104 conidia/mL; both isolates had similar aggressiveness toward these hosts, but differed on Alternanthera (Table 4).
Table 4.
Experimental host ranges of Alternaria species infecting Amaranthaceae.
| Inoculated plants | Disease severity1 and pathogeinicity2 by inoculation with: | Notes6 | |||
|---|---|---|---|---|---|
| Alternaria gomphrenae | Alternaria paragomphrenae3 | Alternaria celosiicola4 | Alternaria alternantherae5 | ||
| MAFF 246769 | MAFF 246768 | MAFF 243058 | |||
| Amaranthaceae | No distinct symptoms were observed on the inoculated leaves with Ago, while Apa often produced small spots without sporulation. Ace is pathogenic to plants in Amaranthoideae. Aal is non-pathogenic to Amaranthus spinosus, but to tribe Celosieae. | ||||
| Amaranthoideae | |||||
| Amarantheae | |||||
| Amaranthus tricolor | 0 | 0.1±0.2 | 4.4±0.6 *** | – | |
| Celosieae | |||||
| Celosia argentea var. cristata | 0 | 0.8±0.3 * | 4.6±0.5 *** | S | |
| C. argentea var. plumosa | 0 | 0.4±0.3 | 4.7±0.3 *** | S | |
| Betoideae | No distinct symptoms were observed on the inoculated leaves with Ace, Ago, and Apa, but Aal was reported as pathogenic to both Beta and Spinacia. | ||||
| Beta vulgaris | 0.3±0.3 | 0 | 0.2±0.3 | S | |
| Chenopodioideae | |||||
| Spinacia oleracea | 0 | 0.1±0.2 | 0.1±0.2 | S | |
| Gomphrenoideae | Distinct leaf spots and defoliations with rich sporulation were observed on Gomphrena inoculated with Ago isolates by 6 dpi. Leaf spots produced by Ago also observed on Alternanthera by 7 dpi but fewer with poor sporulation than on Gomphrena. Apa and Ace has distinct pathogenicity to both Alternanthera and Gomphrena. Aal is partially pathogenic to Alternanthera species at least but not to Gomphrena. | ||||
| Alternanthera sessilis | 0.5±0.7 * | 3.3±0.8 ** | 4.5±0.4 *** | – | |
| Gomphrena globosa | 2.0±0.5 *** | 4.6±0.3 *** | 3.0±0.7 ** | | | |
1 Mean disease severity at 7 d post-inoculation (dpi) rated on a 0–5 scale (0: no visible lesions, 1: <10 % leaf area affected, 2: 11–25 % leaf area affected, 3: 26–50 % leaf area affected, 4: 51–75 % leaf area affected, and 5: >75 % leaf area affected or defoliation). 95 % confidence intervals are also indicated. –: not available. Results for the original plant source of each Alternaria isolate are indicated in bold.
2 Pathogenicity was evaluated by the presence or absence of distinct lesions and sporulation on lesions, and are indicated with asterisks (***: strongly aggressive, showing distinct lesions with rich sporulation; **: weakly aggressive, showing indistinct or fewer distinct lesions with sporulation; *: weakly aggressive to opportunistic, showing few, indistinct lesions with no to rare sporulation; blank: non-pathogenic, showing no distinct lesions nor sporulation).
3 Novel taxa proposed in the taxonomy section.
4 From results reported by Nishikawa & Nakashima (2013).
5 From results reported by Pomella et al. (2007). S: susceptible, I: immune. They also determined Alternanthera philoxeroides and Portulaca halimoides (Portulacaceae) as susceptible hosts, and Alternanthera ficoidea, Amaranthus spinosus, and Hebanthe eriantha (= Pfaffia paniculata; Gomphrenoideae) as immune plants.
6 Fungal names are abbreviated as follows; Ace: Alternaria celosiicola, Ago: A. gomphrenae, Apa: A. paragomphrenae*, and Aal: A. alternantherae.
Distinct reddish spots appeared on Gomphrena after 7 dpi with A. gomphrenae, leading to defoliation, with poor sporulation on lesions even after 30 dpi (Fig. 5A, B). Almost no distinct symptoms caused by A. gomphrenae were observed on the inoculated leaves of Alternanthera or the other examined plants – Amaranthus, Celosia, Beta, and Spinacia – until 30 dpi (Fig. 5C).
Fig. 5.
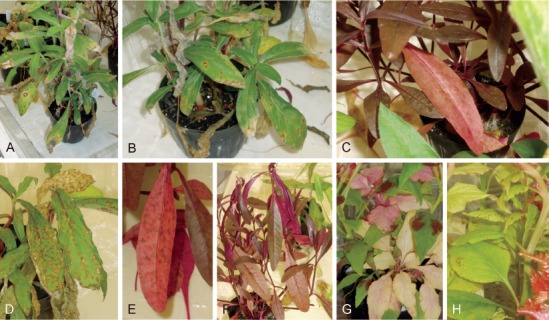
Pathogenicity of two Alternaria species of sect. Alternantherae. A–C. Alternaria gomphrenae (MAFF 246769); A, B. On Gomphrena at 30 d postinoculation (dpi). C. On Alternanthera at 8 dpi. D–H. Alternaria paragomphrenae (MAFF 246768); D. On Gomphrena at 10 dpi. E, F. On Alternanthera at 4–6 dpi. G. On Amaranthus at 11 dpi. H. Celosia at 4 dpi.
Distinct spots caused by MAFF 246768 similar to those of A. gomphrenae were also observed, but were more severe on Gomphrena by 10 dpi (Fig. 5D), while no symptoms were observed on control plants. Indistinct spots frequently appeared on the leaves of Alternanthera inoculated with MAFF 246768 by 2 dpi, and then the leaves were severely defoliated with sporulation by 10 dpi (Fig. 5E, F). Almost no distinct symptoms were observed on the inoculated leaves of the other examined plants until 30 dpi, though they often showed small necrotic spots without sporulation (Fig. 5G, H).
Species infecting Brassicaceae
Three isolates of A. brassicae, MAFF 240791, AC29, and MUCC 1615, were applied by spraying a conidial suspension at an average of 8.4 × 104 conidia/mL (Table 5). Distinct black lesions appeared on the inoculated leaves of all Brassiceae plants, Nasturtium, and Iberis within 2 dpi, and inoculated leaves showed severe rot or defoliation with rich sporulation at 7–10 dpi (Fig. 6A–H). Distinct black spots also appeared on Eutrema at 7 dpi, although these differed in disease severity between applied isolates (Fig. 6I). Lesions on Lobularia and Matthiola were usually indistinct; however, severe leaf blight or rot were observed with rich sporulation at 9–10 dpi (Fig. 6J, K). No distinct symptoms were observed on Aubrieta and Capsella until 30 dpi as on the non-Brassicaceae plants, although poor sporulation was sometimes seen on lower, older leaves (Fig. 6L).
Table 5.
Experimental host ranges of Alternaria species infecting Brassicaceae.
| Inoculated plants | Disease severity1 and pathogenicity2 by inoculation with: | Notes3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Alternaria brassicae | Alternaria brassicicola | Alternaria japonica | |||||||
| AC29 | MAFF 240791 | MUCC 1615 | MAFF 246772 | MAFF 246773 | MUCC 1619 | MAFF 246775 | AC96 | ||
| Brassicaceae | |||||||||
| Alysseae | |||||||||
| Lobularia maritima | 2.4±0.6 ** | 3.6±0.4 ** | 3.3±0.7 ** | 3.5±0.6 ** | 3.0±1.1 ** | 2.8±0.8 ** | 3.1±0.6 ** | 0.2±0.3 * | Small black spots and indistinct leaf blight were observed at 7–10 dpi, usually with rich sporulation. |
| Anchonieae | |||||||||
| Matthiola incana | 3.1±0.4 ** | 3.7±0.5 ** | 2.6±0.5 ** | 4.9±0.2 *** | 4.9±0.2 *** | 4.8±0.3 *** | 4.8±0.3 *** | 3.8±0.4 *** | Black lesions appeared, then whole plants rotted with rich sporulation by 7 dpi, but relatively slight for Abe. |
| Arabideae | |||||||||
| Aubrieta sp. | 0 | 0 | 0.2±0.2 | 0.3±0.2 * | 1.4±0.7 * | 0.3±0.2 * | 1.9±0.5 * | 0.1±0.2 * | Distinct symptoms were rarely observed. Isolate MAFF 246775 of Aja produced necrotic spots without sporulation at 7 pi, nd isolates of Aba often reproduced conidia on stem surfaces without distinct symptoms. |
| Brassiceae | |||||||||
| Brassica juncea | 4.3±0.6 ** | 4.2±0.4 *** | 3.3±0.5 *** | 4.9±0.2 *** | 4.4±0.5 *** | 3.7±0.7 *** | 2.8±0.6 *** | 3.3±0.8 *** | Black spots were produced on leaves of all Brassiceae plants, then whole plants rotted with rich sporulation by 7 dpi. No ignificant differences were found between inoculated species. |
| B. oleracea var. capitate | 4.1±0.4 *** | 3.8±0.3 *** | 4.0±0.5 *** | 4.9±0.1 *** | 4.5±0.6 *** | 5.0 *** | 5.0 *** | 4.7±0.3 *** | |
| B. oleracea var. sabellica | – | – | – | 4.6±0.2 *** | – | – | – | – | |
| B. rapa subsp. Chinensis | – | 4.1±0.5 *** | – | 4.6±0.3 *** | 4.1±0.5 *** | 4.1±0.6 *** | 4.1±0.7 *** | 3.3±0.7 *** | |
| B. rapa subsp. Nipposinica | – | 3.4±0.4 *** | – | 4.7±0.4 *** | 5.0 *** | 5.0 *** | 3.7±0.5 *** | 4.5±0.4 *** | |
| B. rapa subsp. Pekinensis | 4.1±0.2 *** | 4.0±0.3 *** | 4.1±0.2 *** | 4.3±0.4 *** | 4.8±0.3 *** | 4.4±0.6 *** | 2.7±0.7 *** | 3.6±0.6 *** | |
| B. rapa subsp. rapa | 3.4±0.3 *** | 4.0±0.7 *** | 3.9±0.5 *** | 4.6±0.5 *** | 5.0 *** | 5.0 *** | 3.9±0.6 *** | 4.1±0.5 *** | |
| Diplotaxis tenuifolia | 2.8±0.3 ** | 3.4±0.4 *** | 3.9±0.5 *** | 4.7±0.3 *** | 4.4±0.5 *** | – | 3.9±0.6 *** | 3.9±0.6 *** | |
| Eruca vesicaria subsp. sativa | 3.8±0.8 *** | 3.2±0.6 *** | 3.3±0.4 *** | 5.0 *** | 4.4±0.4 *** | 5.0 *** | 4.5±0.4 *** | 4.6±0.3 *** | |
| Raphanus sativus var. sativus | 3.7±0.9 *** | 3.9±0.5 *** | 4.3±0.4 *** | 5.0 *** | 4.3±0.4 *** | 5.0 *** | 4.7±0.4 *** | 4.8±0.3 *** | |
| Cardamineae | |||||||||
| Nasturtium officinale | 3.4±0.5 *** | 3.4±0.5 *** | 3.5±0.3 *** | 4.2±0.7 *** | 2.2±0.8 ** | 4.0±0.5 *** | 4.8±0.3 *** | 1.5±0.6 ** | Black spots were produced on lower leaves, then leaf blight with rich sporulation was observed at 7–10 dpi. |
| Eutremeae | |||||||||
| Eutrema japonicum | 1.5±0.6 * | 3.0±1.6 *** | 3.0 ** | 1.5±0.4 * | 2.0±3.0 ** | 1.1±0.4 * | 1.3±0.7 * | 2.0 * | Leaves inoculated with Abe showed distinct black spots with sporulation at 7 dpi. Leaves inoculated with Aba and Aja showed mostly indistinct tip burn with no to rare sporulation by 10 dpi. |
| Iberideae | |||||||||
| Iberis sempervirens | 1.6±0.5 ** | 1.7±0.4 ** | 3.4±0.5 *** | 4.8±0.2 *** | 3.6±0.9 *** | 2.8±0.6 ** | 1.9±0.4 ** | 2.0±0.4 ** | Small black spots were produced, then the plant easily defoliated with sporulation by 10 dpi; relatively severe for Aba. |
| Lepidieae | |||||||||
| Capsella bursa-pastoris | 0 | 0 | 0 | 0 | 0 | 0.6±0.5 * | 0.5±0.4 * | 0 | No distinct symptoms were typically observed; Aba isolate MUCC 1619 and Aja isolate MAFF 246775 rarely produced necrotic spots without sporulation by 7 dpi. |
| Amaranthaceae | |||||||||
| Beta vulgaris | 0 | 0 | 0 | 0 | 0 | – | – | – | No distinct symptoms observed in this test over 14 dpi, even on Spinacia inoculated with Aba isolate MAFF 246773 ex Spinacia. |
| Chenopodium giganteum | 0 | 0 | 0 | – | – | – | – | – | |
| Spinacia oleracea | – | – | – | 0 | 0 | 0 | – | – | |
| Apiaceae | |||||||||
| Daucus carota | – | 0 | – | 0 | 0 | – | – | – | |
| Asteraceae | |||||||||
| Callistephus chinensis | – | – | – | 0 | 0.2±0.3 | – | – | – | |
| Lactuca sativa | 0 | 0.3±0.2 * | 0 | 0 | 0 | – | 0.2±0.3 | 0 | |
| Convolvulaceae | |||||||||
| Ipomoea nil | 0 | 0 | 0.3±0.3 * | – | – | – | – | – | |
| Cucurbitaceae | |||||||||
| Cucumis sativus | 0.5±0.4 * | 0 | 0 | 0 | 0 | – | – | – | |
| Cucurbita maxima | 0.2±0.3 * | 0 | 0 | – | – | – | – | – | |
| C. pepo | – | – | – | 0.2±0.2 | 0 | – | – | – | |
| Fabaceae | |||||||||
| Phaseolus vulgaris | – | 0 | – | 0 | 0 | – | – | – | |
| Vicia faba | – | 0 | – | 0 | 0 | – | – | – | |
| Vigna unguiculata subsp. Unguiculata | – | – | – | – | – | – | 0.2±0.3 | 0 | |
| Onagraceae | |||||||||
| Clarkia amoena | – | 0 | – | 0 | 0 | – | – | – | |
| Pedaliaceae | |||||||||
| Sesamum indicum | – | – | – | 0 | 0 | – | 0 | 0 | |
| Solanaceae | |||||||||
| Capsicum annuum | – | 0 | – | – | – | – | – | – | |
| Solanum lycopersicum | – | – | – | 0.1±0.2 | 0.3±0.3 | – | – | – | |
| Poaceae | |||||||||
| Zea mays | – | 0 | – | – | – | – | – | – | |
1 Mean disease severity at 7 d post-inoculation (dpi) rated on a 0–5 scale (0: no visible lesions, 1: <10 % leaf area affected, 2: 11–25 % leaf area affected, 3: 26–50 % leaf area affected, 4: 51–75 % leaf area affected, and 5: >75 % leaf area affected or defoliation). 95 % confidence intervals are also indicated. –: not tested. Results for the original plant sources of each Alternaria isolate are indicated in bold.
2 Pathogenicity was evaluated by the presence or absence of distinct lesions and sporulation on lesions, and are indicated with asterisks (***: strongly aggressive, showing distinct lesions with rich sporulation; **: weakly aggressive, showing indistinct or fewer distinct lesions with sporulation; *: weakly aggressive to opportunistic, showing few, indistinct lesions with no to rare sporulation; blank: non-pathogenic, showing neither distinct lesions nor sporulation).
3 Fungal names are abbreviated as follows; Abe: Alternaria brassicae, Aba: A. brassicicola, and Aja: A. japonica.
Fig. 6.

Pathogenicity of Alternaria brassicae (MAFF 240791). A. On Raphanus at 5 d post-inoculation (dpi). B. On Brassica oleracea var. capitata at 5 dpi. C. On B. rapa subsp. pekinensis at 7 dpi. D. On B. rapa subsp. nipposinica at 5 dpi. E. On B. juncea at 5 dpi. F. On Eruca at 7 dpi. G. On Nasturtium at 2 dpi. H. On Iberis at 7 dpi. I. On Eutrema at 10 dpi. J. On Lobularia at 9 dpi. K. On Matthiola at 13 dpi. L. On Aubrieta at 7 dpi.
Three isolates of A. brassicicola (MAFF 246772, MAFF 246773, and MUCC 1619) were applied at an average of 3.2 × 106 conidia/mL (Table 5). Results were similar to those of A. brassicae, with three isolates of A. brassicicola being highly aggressive toward Brassiceae plants, Nasturtium, Iberis, and Matthiola (Fig. 7A–G). On the inoculated leaves of Lobularia, small spots resulting in indistinct leaf blight were observed but with rich sporulation at 10 dpi (Fig. 7H). On the other hand, inoculated Eutrema leaves mostly showed only indistinct tip burn with no to rare sporulation by 18 dpi (Fig. 7I), and the inoculated leaves of Aubrieta and Capsella showed no distinct symptoms. However, sporulation was often observed on suberized stem surfaces of the former species at 7 dpi, and rarely produced necrotic spots on leaves of the latter without sporulation by 7 dpi (Fig. 7J, K). No distinct symptoms appeared on the non-Brassicaceae plants, even on Spinacia, which was the original source of MAFF 246773 (Fig. 7L).
Fig. 7.

Pathogenicity of Alternaria brassicicola (MAFF 246772). A. On Brassica oleracea var. capitata at 7 d post-inoculation (dpi). B. On B. rapa subsp. chinensis at 7 dpi. C. On Raphanus at 7 dpi. D. On Eruca at 7 dpi. E. On Nasturtium at 7 dpi. F. On Iberis at 7 dpi. G. On Matthiola at 7 dpi. H. On Lobularia at 18 dpi. I. On Eutrema at 18 dpi. J. On Aubrieta at 18 dpi. K. On Capsella at 7 dpi. L. On Spinacia at 7 dpi.
Two isolates of A. japonica (MAFF 246775 and AC96) were applied at an average of 1.7 × 106 conidia/mL (Table 5). Similar results as those observed on the former two species were obtained on Brassiceae plants, Nasturtium, Iberis, Lobularia, and Matthiola (Fig. 8A–H). The inoculated leaves of Eutrema, Aubrieta, and Capsella showed no distinct symptoms, although necrotic spots without sporulation were rarely produced at 14 dpi (Fig. 8I–K). No distinct symptoms appeared on the non-Brassicaceae plants (Fig. 8L).
Fig. 8.

Pathogenicity of Alternaria japonica (MAFF 246775). A. On Brassica oleracea var. capitata at 9 d post-inoculation (dpi). B. On B. rapa subsp. rapa at 10 dpi. C. On Raphanus at 10 dpi. D. On Eruca at 4 dpi. E. On Nasturtium at 4 dpi. F. On Iberis at 4 dpi. G. On Lobularia at 9 dpi. H. On Matthiola at 9 dpi. I. On Eutrema at 7 dpi. J. On Aubrieta at 7 dpi. K. On Capsella at 10 dpi. L. On Lactuca at 9 dpi.
Alternaria cumini and a novel species infecting Apiaceae
An isolate of A. cumini (MAFF 246774) was applied at an average of 5.0 × 104 conidia/mL (Table 6). Leaf spots on Cuminum, which was the original source of the fungus used for inoculation, appeared at 2 dpi; leaves became severely blighted and rotten with rich sporulation within 6 dpi (Fig. 9A). On the inoculated leaves of Petroselinum and Anthriscus, mostly small spots or tip burn appeared at 7 dpi, with rare sporulation within 30 dpi (Fig. 9B). No distinct symptoms were observed on the other seven Apiaceae plants, including Coriandrum and Daucus at 30 dpi.
Table 6.
Experimental host ranges of Alternaria species infecting Apiaceae.
| Inoculated plants | Disease severity1 and pathogenicity2 by inoculation with: | References of host range of the other species on Daucus carota | Notes7 | ||||
|---|---|---|---|---|---|---|---|
| Alternaria cumini MAFF 246774 | Alternaria triangularis3 MAFF 246776 | Alternaria petroselini4 MAFF 243057 | Alternaria carotiincultae5 | Alternaria dauci6 | Alternaria radicina5 | ||
| Apiaceae, Apioideae | |||||||
| Apieae | Small spots appeared on leaves of Petroselinum inoculated with Acu, but no sporulation was observed by 30 dpi. No distinct symptoms were observed on leaves inoculated with Atr by 30 dpi. Ape and the other three species are widely and partially pathogenic to the tribe, respectively. The host range of Aca and Ara is identical within the tribe. | ||||||
| Ammi majus | – | 0.1±0.2 | 2.9±1.1 *** | – | – | – | |
| Anethum graveolens | – | – | 4.4±0.7 *** | Weak–Non | HE | Weak–Non | |
| Apium graveolens | 0.1±0.2 | 0.3±0.3 | 3.2±1.2 *** | Weak | L | Weak | |
| Foeniculum vulgare | – | – | 3.3±1.1 *** | Weak | HE | Weak | |
| Petroselinum crispum | 0.3±0.3 * | 0 | 3.8±0.7 *** | Non | L | Non | |
| Bupleureae | Leaves inoculated with Atr showed distinct black spots with sporulation within 7 dpi. Ape is also weakly pathogenic. | ||||||
| Bupleurum rotundifolium | 0 | 3.9±0.7 *** | 2.6±1.1 ** | – | – | – | |
| Careae | No distinct symptoms were observed on leaves inoculated with Acu and Atr, and no other pathogenic species were found. | ||||||
| Carum carvi | 0.3±0.6 | 0.2±0.2 | 0 | Weak–Non | – | Weak–Non | |
| Coriandreae | No distinct symptoms were observed on the inoculated leaves of the two examined species, while Ape, Aca, Ada, and Ara were shown to be pathogenic. | ||||||
| Coriandrum sativum | 0 | 0 | 3.5±0.7 *** | Weak | Hn | Weak | |
| Oenantheae | No distinct symptoms were observed on the inoculated leaves. | ||||||
| Cryptotaenia japonica | 0 | 0 | 0 | – | – | – | |
| Scandiceae | |||||||
| Daucinae | Cuminum leaves inoculated with Acu showed severe leaf blight with rich sporulation at 5 dpi, and Daucus leaves inoculated with examined three species showed no distinct symptoms at 30 dpi. | ||||||
| Cuminum cyminum | 4.5±0.3 *** | – | 5.0 *** | – | – | – | |
| Daucus carota | 0 | 0 | 0.6±0.3 * | HP | HE | HP | |
| Scandicinae | Small spots and tip burn without sporulation was rarely observed on leaves inoculated with Acu at 14 dpi, while Ape and Ada are clearly pathogenic. | ||||||
| Anthriscus cerefolium | 0.4±0.4 * | – | 3.7±0.7 *** | – | Hn | – | |
| Selineae | Small necrotic spots without sporulation were observed at 30 dpi on leaves inoculated with Atr. Only Ape is weakly pathogenic. | ||||||
| Angelica keiskei | 0 | 0.7±1.3 * | 2.2±1.0 ** | – | – | – | |
1 Mean disease severity at 7 d post-inoculation (dpi) rated on a 0–5 scale (0: no visible lesions, 1: <10 % leaf area affected, 2: 11–25 % leaf area affected, 3: 26–50 % leaf area affected, 4: 51–75 % leaf area affected, and 5: >75 % leaf area affected or defoliation). 95 % confidence intervals are also indicated. –: not tested. Results for the original plant sources of each Alternaria isolate are indicated in bold.
2 Pathogenicity was evaluated by the presence or absence of distinct lesions and sporulation on lesions, and are indicated with asterisks (***: strongly aggressive, showing distinct lesions with rich sporulation; **: weakly aggressive, showing indistinct or fewer distinct lesions with sporulation; *: weakly aggressive to opportunistic, showing few, indistinct lesions with no to rare sporulation; blank: non-pathogenic, showing neither distinct lesions nor sporulation).
3 Novel taxa proposed in the taxonomy section.
4 From results reported by Nishikawa & Nakashima (2013).
5 From results reported by Pryor & Gilbertson (2002). HP: highly pathogenic, Weak: weakly pathogenic, Non: non-pathogenic. They also determined that A. carotiincultae, A. petroselini, and A. radicina were weakly or non-pathogenic to Pimpinella anisum (tribe Pimpinelleae) and Pastinaca sativa (tribe Tordylieae).
6 From results reported by Boedo et al. (2012). HE: species exhibiting a relatively high disease index with expanding lesions, Hn: species showing a relatively high disease index with non-expanding lesions, L: species exhibiting a relatively low disease index with non-expanding lesions. They also noted that the inoculated leaves of Pastinaca and non-Apiaceae plants (corn salad and leek) showed a low disease index or were symptomless.
7 Fungal names are abbreviated as follows; Acu: Alternaria cumini, Atr: A. triangularis*, Ape: A. petroselini, Aca: A. carotiincultae, Ada: A. dauci, Ara: A. radicina.
Fig. 9.

Pathogenicity of two Alternaria species on Apiaceae. A, B. Alternaria cumini (MAFF 246774); A. On Cuminum at 7 d post-inoculation (dpi). B. On Anthriscus at 7 dpi. C–H. Alternaria triangularis (MAFF 246776); C, D. On Bupleurum at 8 dpi. E. On stem of Bupleurum at 8 dpi. F. On Bupleurum at 11 dpi. G. On Angelica at 10 dpi. H. On Ammi at 11 dpi.
A conidial suspension of MAFF 246776 ex Bupleurum (A. triangularis) was applied at an average of 6.4 × 104 conidia/mL (Table 6). The inoculated leaves of Bupleurum showed distinct black spots within 5 dpi, and sporulation was abundant on lesions at 7 dpi (Fig. 9C–F); no symptoms were observed on control plants. Small necrotic spots without sporulation were sometimes observed within 30 dpi on the inoculated leaves of Angelica (Fig. 9G). No distinct symptoms were observed on the inoculated leaves of the other seven plants at 30 dpi (Fig. 9H).
A novel species ex Petunia infecting Solanaceae
A conidial suspension of MAFF 246770 (A. cylindrica) was applied at an average of 8.2 × 105 conidia/mL (Table 7). Irregular-shaped lesions appeared abundantly on the inoculated Petunia leaves at 2 dpi, and then lesions quickly expanded and caused severe rot of the whole plant with rich sporulation (Fig. 10A–C), but no symptoms were observed on control plants. Small necrotic spots were observed on the inoculated leaves of Solanum lycopersicum and S. melongena at 2 dpi, and were slightly expanded with little sporulation within 7 dpi (Fig. 10D, E). No distinct symptoms were observed on the other three Solanoideae plants, Nicotiana, and two non-solanaceous plants (Fig. 10F).
Table 7.
Experimental host ranges of Alternaria species on Solanaceae.
| Inoculated plants | Disease severity1 and pathogenicity2 by inoculation with: | References of host range of Alternaria solani5 | Notes | |
|---|---|---|---|---|
| Alternaria cylindrica3 MAFF 246770 | Alternaria crassa4 MAFF 243056 | |||
| Solanaceae | ||||
| Nicotianoideae | No distinct symptoms were observed. | |||
| Nicotiana tabacum | 0.3±0.4 | 0 | Non | |
| Petunioideae | Severe necrosis appeared on leaves inoculated with MAFF 246770 at 2 dpi, which then became rotten with rich sporulation. | |||
| Petunia × atkinsiana | 5.0 *** | 0.2±0.3 * | – | |
| Solanoideae | Small necrotic spots were observed on Solanum inoculated with MAFF 246770 at 2 dpi, which occasionally became slightly expanded with sporulation until 7 dpi. MAFF 246770 showed no pathogenicity to the other examined Solanoideae plants. Host selectivities of A. crassa and A. solani are reported as to each original source plant and Capsicum. | |||
| Capsiceae | ||||
| Capsicum annuum | 0 | 4.2±0.7 *** | M | |
| Datureae | ||||
| Brugmansia × candida | 0 | 2.3±0.7 *** | Non | |
| Physaleae | ||||
| Physalis alkekengi var. franchetii | 0 | 0.3±0.4 | – | |
| Solaneae | ||||
| Solanum lycopersicum | 2.4±0.7 * | 1.2±0.3 * | S | |
| S. melongena | 2.9±0.9 * | 0.4±0.4 | S | |
| Fabaceae | No distinct symptoms were observed over 30 dpi on leaves of non-host plants inoculated with MAFF 246770. | |||
| Vigna unguiculata | 0 | – | – | |
| Poaceae | ||||
| Zea mays | 0 | – | – | |
1 Mean disease severity at 7 d post-inoculation (dpi) rated on a 0–5 scale (0: no visible lesions, 1: <10 % leaf area affected, 2: 11–25 % leaf area affected, 3: 26–50 % leaf area affected, 4: 51–75 % leaf area affected, and 5: >75 % leaf area affected or defoliation). 95 % confidence intervals are also indicated. –: not tested. Results for the original source plant species (or close relatives) of each Alternaria species are indicated in bold.
2 Pathogenicity was evaluated by the presence or absence of distinct lesions and sporulation on lesions, and are indicated with asterisks (***: strongly aggressive, showing distinct lesions with rich sporulation; **: weakly aggressive, showing indistinct or fewer distinct lesions with sporulation; *: weakly aggressive to opportunistic, showing few, indistinct lesions with no to rare sporulation; blank: non-pathogenic, showing neither distinct lesions nor sporulation).
3 Novel taxa proposed in the taxonomy section.
4 From results reported by Nishikawa & Nakashima (2013).
5 From results reported by Cardoso (2014). S: susceptible, M: caused mild symptoms, Non: non-pathogenic (symptoms or pathogen structures absent). Cardoso’s results also confirmed virulences of A. solani toward three asteraceous species (Ageratum conyzoides, Erigeron bonariensis, and Galinsoga parviflora), and Rumex acetosa (Polygonaceae).
Fig. 10.

Pathogenicity of Alternaria cylindrica (MAFF 246770). A–C. On Petunia; A, B. At 3 d post-inoculation (dpi). C. At 7 dpi. D. On Solanum lycopersicum at 6 dpi. E. On S. melongena at 7 dpi. F. On Brugmansia at 9 dpi.
Alternaria iridicola infecting Iridaceae
Two isolates of A. iridicola (MAFF 246890 and MAFF 246771) were applied at an average of 3.2 × 105 conidia/mL, and both isolates showed similar results (Table 8). Distinct leaf spots appeared on Iris spp., except for I. ensata, at 7 dpi, and then the inoculated leaves became severely blighted with rich sporulation (Fig. 11A, B). As for I. ensata, neither distinct symptoms nor sporulation were observed within 14 dpi (Fig. 11C). Small yellow spots and slightly yellowing spots with no or poor sporulation were observed at 7 dpi on Gladiolus and Crocus, respectively (Fig. 11D, E). No distinct symptoms were observed on Freesia and Asparagus, while yellow spots and tip burn without sporulation were sometimes observed on leaves of Allium at 14 dpi (Fig. 11F–H).
Table 8.
Experimental host range of Alternaria iridicola.
| Inoculated plants | Disease severity1 and pathogenicity2 by inoculation with: | Notes | |
|---|---|---|---|
| MAFF 246890 | MAFF 246771 | ||
| Amaryllidaceae | Small yellow spots and tip burn without sporulation were rarely observed at 14 dpi. | ||
| Allium fistulosum | 0.7±0.7 * | 0.3±0.3 * | |
| Asparagaceae | No distinct symptoms observed by 14 dpi. | ||
| Asparagus officinalis | 0.1±0.3 | – | |
| Iridaceae | |||
| Crocoideae | Slightly yellowing on tips with occasional sporulation were observed. | ||
| Crocus sp. | 1.0±0.8 * | 1.7±0.7 * | |
| Iridoideae | Leaf spots and sever blight with rich sporulation on I. laevigata and Iris × hollandica were observed at 7 dpi, but I. ensata var. spontanea never showed any symptoms over 14 dpi. | ||
| Iris ensata var. spontanea | 0.6±0.5 | 0.3±0.3 | |
| I. laevigata | 3.5±1.3 *** | 4.0±0.8 *** | |
| Iris × hollandica | 4.1±0.6 *** | 4.4±0.5 *** | |
| Ixioideae | Small yellow spots were often produced on Gladiolus by 14 dpi, but never expanded and sporulated. No distinct symptoms were observed on Freesia by 14 dpi. | ||
| Freesia refracta | 0 | 0 | |
| Gladiolus sp. | 0.3±0.5 | 0.3±0.3 | |
1 Mean disease severity at 7 d post-inoculation (dpi) rated on a 0–5 scale (0: no visible lesions, 1: <10 % leaf area affected, 2: 11–25 %, 3: 26–50 %, 4: 51–75 %, and 5: >75 % or defoliated). 95 % confidence intervals also indicated. –: not tested. Results of the original source plant genus of each Alternaria species are indicated in bold.
2 Pathogenicity was evaluated by presence or absence of distinct lesion and sporulation on lesion, and indicated asterisks (***: strongly aggressive, showing distinct lesions with rich sporulation, **: weakly aggressive, showing indistinct or fewer distinct lesions with sporulation, *: weakly aggressive to opportunistic, showing fewer indistinct lesions with no to rare sporulation, blank: non-pathogenic, showing distinct lesions nor sporulation).
Fig. 11.

Pathogenicity of Alternaria iridicola (MAFF 246890). A. On Iris laevigata at 6 d post-inoculation (dpi). B. On Iris × hollandica at 14 dpi. C. On I. ensata at 14 dpi. D. On Gladiolus at 14 dpi. E. On Crocus at 6 dpi. F. On Freesia at 14 dpi. G. On Asparagus at 14 dpi. H. On Allium at 14 dpi.
Alternaria porri on non-host plants
Obtained A. porri isolates from non-host plants, such as isolates AC2 ex Viola (Violaceae), AC6 ex Calibrachoa (Solanaceae), and MUCC 1702 ex Eustoma (Gentianaceae), were used to inoculate each original host and related plants by spraying with a conidial suspension concentrated at an average of 2.0 × 105 conidia/mL. No isolates showing pathogenicity toward non-alliaceous plants including each source host plant were found.
Taxonomy
Eighty-five Japanese Alternaria isolates were collected, and found to represent 26 species, of which three were new to science. Each species is described for each Alternaria section in alphabetical order below.
Section Alternantherae D.P. Lawr. et al., Mycologia 105: 540. 2013.
Four species were recognized in this section (Lawrence et al. 2013, Woudenberg et al. 2013), and Gannibal (2018) added two additional species. All taxa and phylogenetically unresolved species (A. crassoides and A. pimpriana) were former members of the genus Nimbya, and parasitic toward Amaranthaceae plant hosts (Simmons 1989, 1995b, 2004). In this section, two known species and one novel species were found.
Alternaria celosiicola Jun. Nishikawa & C. Nakash., J. Phytopathol. 161: 606. 2013. Fig. 12.
Fig. 12.
Morphological features of Japanese isolates of Alternaria celosiicola (MAFF 243058) on potato-carrot agar medium. A–E. Conidia and lumina. F. Colored beak. G. Conidiophores. H. Natural symptoms on Celosia. Scale bars = 25 µm.
Synonyms: Nimbya celosiae E.G. Simmons & Holcomb, Mycotaxon 55: 144. 1995.
Alternaria celosiae (E.G. Simmons & Holcomb) Lawrence, Park & Pryor, Mycol. Progr. 11: 811. 2012, nom. illeg. (later homonym; ICN Art. 53.1). non Alternaria celosiae (Tassi) O. Săvul., Herb. Mycol. Rom.: fasc. 30, no. 1489. 1950.
Alternaria cristata D.P. Lawr., M.S. Park & B.M. Pryor, Mycol. Progr. 13 (2): 259. 2014, nom. nud., ICN Art. 52.1.
Typus: USA, Louisiana, on Celosia cristata, Jul. 1993, G.E. Holcomb, holotype BPI 803020, isotype IMI 369150 and IMI 369153, culture ex-type EGS42.013.
Specimens and isolates examined: Japan, Kanagawa Prefecture, Fujisawa, on leaves of Celosia argentea var. plumosa, 26 Jun. 2006, S. Masugi & Y. Makizumi, MUMH 11676 and MUMH 11701, living culture MAFF 243058.
Morphological characters on V8 medium: Previously reported in Nishikawa & Nakashima (2013). Morphology observed on PCA medium similar to that observed on V8 medium (Table 3).
Colony characteristics on PDA after 7 d at 25 °C: Fast-growing, reaching 71.2 ± 0.9 mm diam; aerial hypha cottony, pale green to grayish green, with white margins; reverse center black to dark green; sporulation sparse; diffusible pigment absent (Nishikawa & Nakashima 2013).
Sexual morph: Not observed.
Natural hosts: Celosia (Amaranthaceae).
Symptoms: Leaf spots on Celosia are circular to subcircular, 2–10 mm diam, brown to dark brown with distinct reddish margin, and often surrounded by a yellowish halo, becoming confluent (Nishikawa & Nakashima 2013).
Experimental host range: Pathogenic to Celosia, Amaranthus, Alternanthera, and Gomphrena, but not to Beta and Spinacia (Nishikawa & Nakashima 2013).
Distribution: USA (Simmons 1995b), China (Zhao & Zhang 2005), and Japan (Nishikawa & Nakashima 2013).
Distinctive features: Conidia are larger than those of other related species in sect. Alternantherae; conidial bodies usually exceeded 100 × 20 µm, and beaks exceed 200 µm long. Conidial bodies commonly consist of a distosepta-like internal wall structure with octagonal lumina. This species is widely pathogenic not only to Celosia, but also to Amaranthus, Alternanthera, and Gomphrena (Table 4), and is phylogenetically recognizable via its ITS (Fig. 2), gapdh, and act sequence data (data not shown).
Notes: Lawrence et al. (2012) transferred this species from the genus Nimbya to Alternaria based on phylogenetic analysis, but the proposed name – A. celosiae (E.G. Simmons & Holcomb) Lawrence, Park & Pryor – resulted in a later homonym of A. celosiae (Tassi) O. Săvul. Although Lawrence et al. (2014) renamed the epithet of A. celosiae to Alternaria cristata Lawrence, Park & Pryor (MB 803181), this name is a later synonym of A. celosiicola described by Nishikawa & Nakashima (2013).
Alternaria gomphrenae Togashi, Bull. Imp. Coll. Agric. Forest. Morioka, Japan 9: 6. 1926. Figs 13, 14.
Fig. 13.
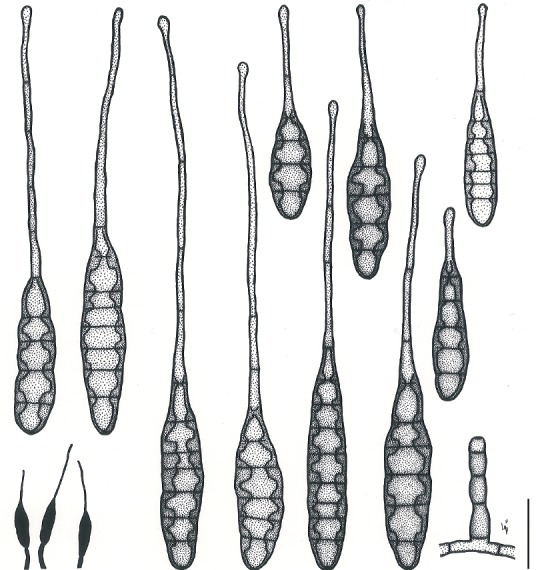
Illustrations of Alternaria gomphrenae (MAFF 246769). Morphology of conidia and conidiophores, and sporulation patterns (opaque) on V8 juice agar medium. Scale bar = 25 µm.
Fig. 14.

Morphological features of Japanese isolates of Alternaria gomphrenae (MAFF 246769) on V8 juice agar medium. A–H. Conidia and lumina. I. Colored beak. J. Conidiophores. K. Dried culture specimen ex MAFF 246769 (epitype: TNS-F-85451). L. Natural symptoms on the specimen of Gomphrena globosa (isoepitype: MUMH 11685). M. Culture on potato-dextrose agar medium (upper = surface, lower = reverse). N, O. Lectotype specimen, TNS-F-243868. P, Q. Conidia on lectotype. Scale bars (A–J, P, Q) = 25 µm.
Synonyms: Nimbya gomphrenae (Togashi) E.G. Simmons, Sydowia 41: 324. 1989.
Pseudocercospora gomphrenicola Chidd., Sci. Cult. (Calcutta) 22: 511. 1957.
Typus: Japan, Kyoto Prefecture, Kitashirakawa, on leaves of Gomphrena globosa (not specified; syntype specimens are assigned as lectotype and paralectotype, respectively, in this study). Lectotype designated here: Japan, Kyoto Prefecture, Kitashirakawa, on leaves of G. globosa, 24 Aug. 1924, K. Togashi, TNS-F-243868 [MBT 385025]; Paralectotype: Japan, Kyoto Prefecture, Kitashirakawa, on leaves of G. globosa, 5 Aug. 1925, K. Togashi, TNS-F-243861; ibid., 10 Aug. 1925, K. Togashi, TNS-F-243862; ibid., 4 Dec. 1925, K. Togashi, TNS-F-243866; ibid., 19 Aug. 1924, K. Togashi, TNS-F-243867; ibid., 22 Jun. 1925, K. Togashi, TNS-F-243872; ibid., 7 Aug. 1924, T. Hemmi & K. Togashi, TNS-F-243873; ibid., 17 Aug. 1924, K. Togashi, TNS-F-243875. Epitype designated here: Japan, Shizuoka Prefecture, Kakegawa, on leaves of G. globosa, 16 Oct. 2011, J. Nishikawa, TNS-F-85451 (dried culture of MAFF 246769) [MBT 385026], isoepitype MUMH 11685, culture ex-isoepitype MAFF 246769.
Additional materials examined: Japan, Kyoto Prefecture, Kitashirakawa, on leaves of G. globosa, 5 Aug. 1925, K. Togashi, TNS-F-243861; ibid., 10 Aug. 1925, K. Togashi, TNS-F-243862; ibid., 4 Dec. 1925, K. Togashi, TNS-F-243866; ibid., 22 Jun. 1925, K. Togashi, TNS-F-243872; ibid., 7 Aug. 1924, T. Hemmi & K. Togashi, TNS-F-243873; ibid., 17 Aug. 1924, K. Togashi, TNS-F-243875.
Morphological characters on V8 medium: Conidiophores short and broad, 25–65 × 5–8 µm. Conidia usually solitary, brown to dark brown, subcylindrical to long obclavate, 50–287 µm in total length, surface smooth; conidial bodies 35–77 × 10–17 µm, with 3–9 transverse and 0–1 longitudinal septa consisting of distosepta. The lumina usually distinct, octagonal to round; filamentous beaks usually straight, subhyaline to pale brown, sometimes multiseptated, unbranched, conspicuously border the conidial body, often knobbed at the apex, 13–216 × 2–4 µm. Conidial bodies on lectotype specimens (TNS-F-243868) 46–94 × 10–16 µm, with 4–10 transverse and no longitudinal septa. Morphology on PCA medium and lesions similar to those observed on V8 medium (Table 3).
Colony characteristics on PDA after 7 d at 25 °C: Slow to moderate-growing, reaching an average of 48 ± 1.8 mm diam; aerial hypha cottony, grayish green to white, with inconspicuous margins; reverse center pale brown to reddish orange; sporulation sparse.
Sexual morph: Not observed.
Natural hosts: Gomphrena (Amaranthaceae).
Symptoms: Leaf and stem spots on G. globosa are circular to elliptical, 2–10 mm diam, pale brown with reddish margins, and become enlarged and confluent, resulting in leaf blighting.
Experimental host range: Selectively pathogenic to Gomphrena; weakly pathogenic or opportunistic to Alternanthera; nonpathogenic to Celosia and the other examined Amaranthaceae plants (Table 4).
Distribution: In Asia (Cambodia, China, India, Indonesia, Japan, Malaysia, Myanmar, and Sri Lanka), as well as North and Latin America (Cuba, Jamaica, Trinidad and Tobago, and USA) (Yoshii 1933, Ellis 1976, Simmons 1989, Zhao & Zhang 2005).
Distinctive features: Conidia are long obclavate, shorter, and narrower (usually not exceeding 100 × 20 µm) than other species infecting Amaranthaceae. They rarely have longitudinal septa, with octagonal lumina and colored beaks. This species is selectively pathogenic to Gomphrena, and recognizable phylogenetically via its ITS (Fig. 2), gapdh, rpb2, and act sequences (data not shown).
Notes: This species is the causal pathogen of leaf spot on Gomphrena, and was first described by Togashi (1926) in Japan. In this description, Togashi also described 10 specimens, but did not specify the holotype. Fortunately, eight of these specimens were preserved as “TYPUS” in TNS; therefore, we re-examined these syntype specimens and selected TNS-F-243868 as a lectotype in the present study. However, we confirmed rich sporulation of A. gomphrenae, often together with those of a small-spored Alternaria species, and found that Togashi, in fact, failed to establish pure cultures, much less complete inoculation testing (Togashi 1926, Yoshii 1933, Simmons 1989, 1995b). Therefore, we also designated an epitype specimen.
Alternaria paragomphrenae Jun. Nishikawa & C. Nakash., sp. nov. MycoBank MB829109. Figs 15, 16.
Fig. 15.
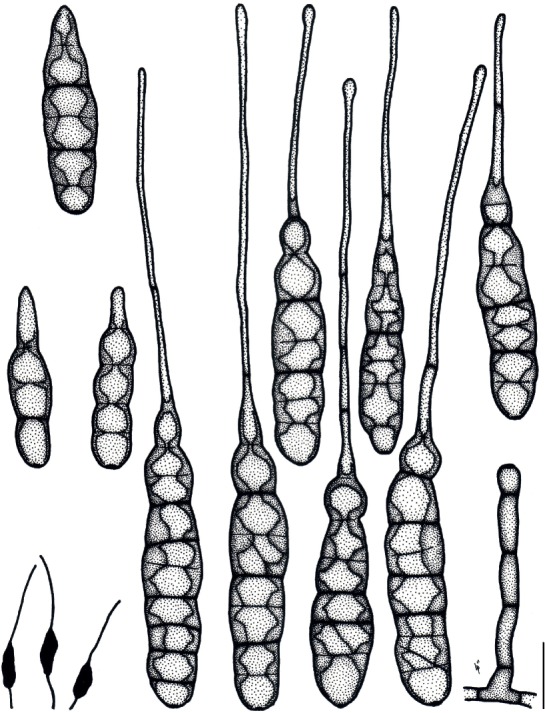
Illustrations of Alternaria paragomphrenae (MAFF 246768). Morphology of conidia and conidiophores, and sporulation patterns (opaque) on V8 juice agar medium. Scale bar = 25 µm.
Fig. 16.

Morphological features of Alternaria paragomphrenae (ex-holotype culture MAFF 246768) on V8 juice agar medium. A–H. Conidia and lumina. I. Conidiophores. J. Colored beak. K. Culture on potato-dextrose agar medium (upper = surface, lower = reverse). L. Natural symptoms on Gomphrena haageana. M. Dried culture specimen ex MAFF 246768 (holotype: TSN-F-85449). N. Isotype specimen, MUMH 242310. Scale bars (A–J) = 25 µm.
Etymology: Named because of its close resemblance to Alternaria gomphrenae, both in conidial morphology and host range.
Diagnosis: Conidial bodies are cylindrical, commonly less than 100 µm in length and exceeded 20 µm in width, sometimes with longitudinal septa forming octagonal lumina, with colored beaks. Pathogenicity of the species is selective to Gomphrena and Alternanthera. It is phylogenetically recognizable among sect. Alternantherae via act, Alt a 1, gapdh, rpb2, and tef1 sequences.
Leaf and stem spots appear on G. haageana (Amaranthaceae), and are pale brown with a small, grayish eye in the center surrounded by reddish margins. They are circular to elliptical, 2–6 mm diam, scattered, show water-soaked enlargement, and become confluent resulting in leaf blighting. On V8 medium, conidiophores are short and thick, 27–81 × 5–7 µm. Conidia usually solitary, pale to brown, ellipsoid to cylindrical, 58–409 µm in total length, with a smooth surface; conidial bodies 48–98 × 17–33 µm, with 3–7 transverse and 0–4 longitudinal septa consisting of distosepta, constricted at each transverse septa. The lumina distinct to indistinct, octagonal to round; filamentous beaks straight to curved, subhyaline to pale brown, sometimes multiseptated, unbranched, conspicuously border the conidial body, 25–316 × 3–5 µm, elongated on cultures rather than on lesions. Morphology on PCA similar to that observed on V8 medium; conidiophores 43–125 × 6–9 μm, conidia 60–294 µm in total length, conidial bodies 60–111 × 15–25 μm, with 2–9 transverse and 0–3 longitudinal septa, beaks 14–208 × 2–5 µm. On lesions, conidiophores 26–99 × 5–7 µm, conidia 35–173 µm in total length, conidial bodies 25–99 × 8–26 µm, with 1–9 transverse and 0–2 longitudinal septa, beaks 14–87 × 3–4 µm.
Typus: Japan, Shizuoka Prefecture, Hamamatsu, Hamakita, on leaves of Gomphrena haageana, 14 Sep. 2004, J. Nishikawa, holotype TNS-F-85449 (a dried culture specimen ex MAFF 246768), culture ex-holotype MAFF 246768 = MUCC 1683, isotype MUMH 242310, GenBank accession number gapdh: LC482000, rpb2: LC476783, tef1: LC480207, Alt a 1: LC481610, act: LC481858.
Experimental host range: Selectively pathogenic to subfamily Gomphrenoideae (Gomphrena and Alternanthera), but sometimes weakly to Celosia and the other examined Amaranthaceae plants (Table 4).
Distribution: Only known from the type collection.
Colony characteristics on PDA after 7 d at 25 °C: Moderate-growing, reaching an average of 59 ± 2.4 mm diam; aerial hypha cottony, pale gray to white, with inconspicuous margins; reverse center yellowish to reddish orange; sporulation sparse.
Sexual morph: Not observed.
Notes: This species demonstrates specific pathogenicity to Gomphrena in common with A. gomphrenae, and is also more aggressive to Alternanthera than A. gomphrenae (Table 4). This species is phylogenetically supported by DNA sequence data combined with gapdh, tef1, and rpb2, but close to A. celosiicola rather than A. gomphrenae (Fig. 1). Based on its shorter and wider bodies, this species is morphologically distinct from these two relevant species (Table 3).
Section Alternaria D.P. Lawr. et al., Mycologia 105: 538. 2013.
This section was morphologically characterized by catenate, small spores, which were typified by those of A. alternata (Lawrence et al. 2013, Woudenberg et al. 2015). Eleven species with three formae speciales of A. alternata and one species complex (A. arborescens) were recognized by Woudenberg et al. (2015). This section also includes important host-selective toxin producers, and they were recognized as distinct species or formae speciales of A. alternata (Woudenberg et al. 2015). Four species with two formae speciales and a novel species from Japan are described in the present study. Additionally, A. iridicola is newly assigned to this section.
Alternaria alstroemeriae E.G. Simmons & C.F. Hill, in Simmons, CBS Biodiversity Ser. (Utrecht) 6: 444. 2007. Fig. 17.
Fig. 17.

Morphological features of Japanese isolates of Alternaria alstroemeriae (MAFF 241374) on potato-carrot agar medium. A–G. Conidia. H. Submerged sporulation in media. I. Conidiophores. Scale bars = 25 µm.
Typus: Australia, on leaves of Alstroemeria sp., Jul. 2005, C.F. Hill, holotype BPI 877375 (dried culture ex EGS 52.068), culture ex-type CBS 118809 = EGS 52.068.
Additional material examined: Japan, Nagano Prefecture, Matsumoto, on leaves of Alstroemeria sp., Jan. 2008, N. Yamagishi, living culture MAFF 241374.
Morphological characters on PCA medium: Previously reported in Yamagishi et al. (2009).
Colony characteristics on PDA after 7 d at 25 °C: Fast-growing, reaching 74.6 ± 1.6 mm diam (Nishikawa & Nakashima 2013); aerial hypha sparse, olive brown to black, with indistinct margins; reverse center black to dark green; sporulation abundant on conidiophores arising from aerial and submerged hypha; diffusible pigment absent.
Sexual morph: Not observed.
Natural host: Alstroemeria (Alstroemeriaceae).
Symptoms: Leaf spots are circular to subcircular, 3–12 mm diam, and are dark brown to black. The same spots also form on stems, followed by defoliation.
Experimental host range: The Japanese isolate shows a restricted host range; it is pathogenic to Alstroemeria, but not to the other plants formerly classified as Liliaceae, including Lilium, Tulipa, Allium, Asparagus, and Hyacinthus (Nishikawa & Nakashima 2013).
Distribution: Limited to Australia (Simmons 2007), and Japan (Yamagishi et al. 2009, Nishikawa & Nakashima 2013).
Distinctive features: Mature and basal conidia are often subcylindrical, rarely with longitudinal septa; conidia appear in short chains (never exceeding 10) in 7 d; sporulation occurs in part when submerged in agar substrate. It is phylogenetically distinguishable from the other species of this section via its gapdh, rpb2, tef1, Alt a 1, and endoPG sequences (Figs 1, 3), but not via ITS (Fig. 2) and act sequences (data not shown).
Alternaria alternata (Fr.) Keissl., Beih. Bot. Centralbl., Abt. 2, 29: 434. 1912. Fig. 18.
Fig. 18.

Japanese isolates of Alternaria alternata. A–K. Conidia and conidiophores on potato-carrot agar medium ex MUCC 1610 (A, B), MUCC 1611 (C, D), MUCC 1616 (E, F), and MAFF 239887 (G–K). L–O. Culture on potato-dextrose agar medium (upper = surface, lower = reverse) of MUCC 1610 (L), MUCC 1611 (M), MUCC 1616 (N), and MAFF 239887 (O). P–R. Natural symptoms on Impatiens (P), Antirrhinum (Q), and Pelargonium (R). Scale bars (A–J) = 25 µm.
Synonyms: Torula alternata Fr., Syst. Mycol. (Lundae) 3: 500. 1832, nom. sanct.
Alternaria tenuis Nees, Syst. Pilze (Würzburg): 72. 1817.
Additional synonyms fully provided in Woudenberg et al. (2015) although A. viniferae was doubtful as described below.
Typus: Neotype, on fragments of a pithy stem, C.G.D. Nees von Esenbeck, L 910, 262-129 (designated in Simmons 1967); Epitype, India, on Arachis hypogaea, 1 Dec. 1980, E.G. Simmons, IMI 254138 (designated in de Hoog & Horré 2002), culture exepitype CBS 916.96 = ATCC 66981 = EGS 34.016.
Additional materials examined: Japan, from seeds of Vigna radiata, 1998, T. Sato, MUMH 11693, living culture MAFF 239887; Nagano Prefecture, Azumino, on leaves of Impatiens hawkeri, 28 Aug. 2006, J. Nishikawa, living culture MUCC 1610; Shizuoka Prefecture, Kakegawa, on leaves of Antirrhinum majus, 28 May 2008, J. Nishikawa, MUMH 11682, living culture MUCC 1611; Kanagawa Prefecture, Nakai, on leaves of Pelargonium hortorum, 29 Sep. 2004, J. Nishikawa, MUMH 11672, living culture MUCC 1616; Shizuoka Prefecture, Kakegawa, on leaves of Primula × polyantha, 6 Nov. 2004, J. Nishikawa, MUMH 11674, living culture MUCC 1617; Shizuoka Prefecture, Kakegawa, on leaves of Solanum lycopersicum, 28 Jun. 2011, J. Nishikawa, living culture AC82; Tokyo, Chiyoda, from seeds of V. radiata, Dec. 2012, T. Sato, living culture MAFF 243775; Kanagawa Prefecture, on leaves of Pyrus aromatica, 1958, S. Toyota, living culture MAFF 305014; on leaves of Pyrus sp.?, M. Kusunoki, living culture MAFF 410775.
Morphological characters on PCA medium: Conidiophores solitary, subcylindrical, unbranched, straight or geniculate, thin, 15–93 × 3–5 μm, sometimes proliferating sympodially. Conidia form as complex, long chains of 10–22, commonly with lateral branches, highly varied, ovoid to ellipsoid, pyriform or obclavate, pale brown to brown, usually smooth; conidial bodies 11–50 × 7–18 μm and 25 × 12 μm on average, commonly not exceeded 50 μm long, with 1–7 transverse and 0–5 longitudinal septa, slightly constricted at the median and some transverse septa. Secondary conidiophores (false beaks) appear at the apical end of conidia, short and mostly single-celled, but often unstable in length (some in isolate MAFF 239887 elongated, reaching 19–110 μm).
Colony characteristics on PDA after 7 d at 25 °C: Fast-growing, reaching an average of 77.3 ± 1.3 mm diam; aerial hypha cottony, sometimes sparse, variable in color, pale gray, grayish green to dark green, with white margins; reverse center black to dark green; sporulation abundant; diffusible pigment absent.
Sexual morph: Not observed.
Natural hosts: Multiple genera in multiple families serve as hosts, and it is often saprophytic; 692 host records were found in USDA Fungal databases (Farr & Rossman 2018), and 32 diseases in the database of plant diseases in Japan, including three pathotype strains (http://www.gene.affrc.go.jp/databasesmicro_pl_diseases_en.php).
Symptoms: Necrotic spots on Impatiens are sometimes circular and often irregular, measuring 1–8 mm diam. Leaf spots on Antirrhinum are usually fairly circular, measuring 5–10 mm diam, developing into coalesced lesions that are gray with pale brown margins. Leaf and stem spots on Pelargonium seedlings are irregular, then represented as leaf blight. As for isolates MAFF 239887 and MAFF 243775 obtained from Vigna, lesions appearing from the roots to the hypocotyls of sprouts appear black and rotten (Sato et al. 2014, Sato 2015), and angular to irregular spots are produced on inoculated true leaves (Sato, pers. comm.).
Distribution: Ubiquitous.
Distinctive features: Small conidia, conidial bodies rarely exceed 50 µm long, and are formed in long chains (mostly over 20 conidia), frequently with lateral branches. Secondary conidiophores are commonly short, consisting of 1–2 cells.
Notes: Woudenberg et al. (2015) synonymized 35 names under this species based on their multi-locus phylogeny. Those synonyms include important host selective species, such as A. mali, A. limoniasperae, and A. toxicogenica, which were newly assigned as formae speciales (with pathotypes) of the species: f. sp. mali, f. sp. citri pathotype rough lemon, and f. sp. citri pathotype tangerine, respectively. Alternaria viniferae was synonymized by Woudenberg et al. (2015); however, this classification is problematic because of the original description using a gapdh and Alt a 1-based phylogeny, as well as A. alstroemeriae-like morphology (Tao et al. 2014), which are highly distinguishable from those of A. alternata.
Alternaria cylindrica Jun. Nishikawa & C. Nakash., sp. nov. MycoBank MB829136. Figs 19, 20.
Fig. 19.
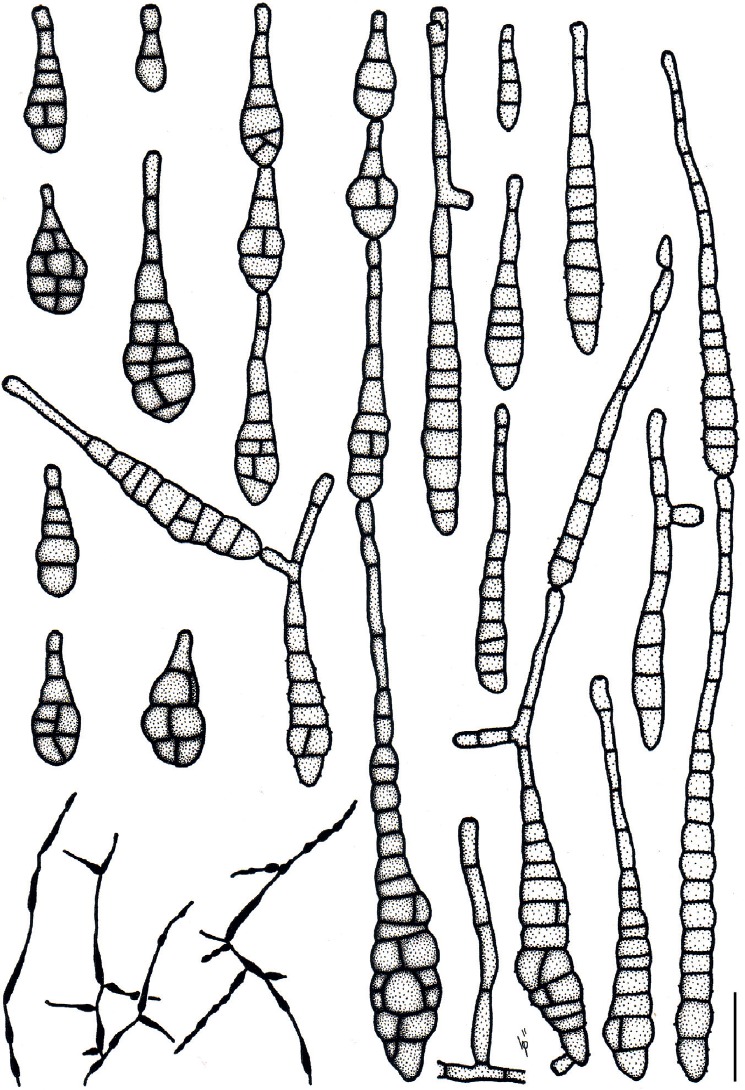
Illustrations of Alternaria cylindrica (ex-holotype culture MAFF 246770). Morphology of conidia and conidiophores, and sporulation patterns (opaque) on V8 juice agar medium. Scale bar = 25 µm.
Fig. 20.

Morphological features of Japanese isolates of Alternaria cylindrica (ex-holotype culture MAFF 246770). A–I. Conidia and conidiophores on potato-carrot agar medium. J. Conidia on V8 juice agar medium. K. Conidia on lesion. L. Culture on potato-dextrose agar medium (upper = surface, lower = reverse). M, N. Natural symptoms on Petunia. O. Dried culture specimen ex MAFF 246770 (holotype: TNS-F-85450). P. Isotype specimen. Scale bars (A–K) = 25 µm.
Etymology: Named after the Latin “cylindricus”, referring to the shape of the conidia, which are cylindrical.
Diagnosis: Long and narrow, cylindrical conidia with few longitudinal septa, which are produced abundantly in long chains and are quite distinctive among members of the genus. Obclavate conidia such as typically seen in sect. Alternaria are often produced at the apex and sides of chains. Pathogenicity is selective to Petunia among members of the Solanaceae. It is phylogenetically close to A. alternata and the A. arborescens species complex.
Leaf spots appear on Petunia, which are dark brown to black with a pale brown eye in the center. They are circular to irregular,measuring 4–7 mm diam, are scattered, and become enlarged and confluent, resulting in blighting of leaves. On V8 medium, conidiophores narrow and short to moderately long, 28–90 × 4–6 µm. Conidia mostly in chains of 5–9 conidia (moderately long chain), lateral branches frequently present in 5–7 d. Conidia subhyaline to pale brown, obclavate and cylindrical to long and narrow ovoid, 11–214 µm in total length, with 0–21(–33) transverse and 0–7 (relatively uncommon) longitudinal septa. Conidial bodies 11–156 × 5–20 µm with 0–13(–25) transverse and 0–7 (relatively uncommon) longitudinal septa, usually smooth surface but very occasionally faintly rough. Secondary conidiophores (false beaks) elongated up to 120 × 3–6 µm. On lesions, conidiophores 30–79 × 5–7 µm. Conidia typically 21–192 µm in total length; conidial bodies 11–89 × 6–18 µm with 1–14 transverse and 0–7 longitudinal septa; secondary conidiophores up to 121 × 3–6 µm. Morphology grown on PCA medium similar to that observed on V8 medium: conidiophores 29–98 × 3–6 μm; conidia 18–270(–340) µm in total length, with 0–30(–43) transverse and 0–4 longitudinal septa; conidial bodies 10–115 × 5–14 μm, with 0–19 transverse and 0–4 longitudinal septa; secondary conidiophores up to 225 × 3–5 µm.
Typus: Japan, Shizuoka Prefecture, Kakegawa, on leaves of Petunia × atkinsiana, 16 Dec. 2006, J. Nishikawa, holotype TNS-F-85450 (a dried culture specimen ex MAFF 246770), isotype MUMH 11678 and MUMH 11703, culture ex-holotype MAFF 246770, GenBank accession number ITS: LC440584, gapdh: LC482006, rpb2: LC476791, tef1: LC480211, Alt a 1: LC481616, endoPG: LC480951, act: LC481867.
Experimental host range: Selectively pathogenic to Petunia among members of the examined Solanaceae plants, but remarkable lesions with no sporulation were frequently observed on the inoculated leaves of Solanum (Table 7).
Distribution: Only known from Japan.
Colony characteristics on PDA after 7 d at 25 °C: Moderate-growing, reaching 58.4 ± 0.7 mm diam; aerial hypha cottony, pale gray to grayish or bluish green, with white margins; reverse center dark green to black; sporulation sparse; diffusible pigment absent.
Sexual morph: Not observed.
Notes: Takano (2005) reported a similar disease on Petunia in Japan, and he identified the pathogen as A. longissima, which was commonly known as a saprophytic fungus of numerous plants and was characterized as having cercosporoid scolecospores with few longitudinal septa (Deighton & MacGarvie 1968, Ellis 1971). Alternaria longissima has since been transferred to the genus Prathoda by Simmons (2007), which was supported by molecular phylogenetic analysis indicating that the species should not be treated as an Alternaria species (Pryor & Gilbertson 2000). Both our examined isolate and those in Takano (2005) were nearly morphologically identical to each other; however, our examined isolate obviously belongs to a species in the sect. Alternaria based on phylogenetic analysis (Figs 1–3). Although these isolates need to be re-examined, they were probably previously misidentified.
Alternaria gaisen Bokura, J. Pl. Protect. (Tokyo) 11: 490. 1924. Fig. 21.
Fig. 21.
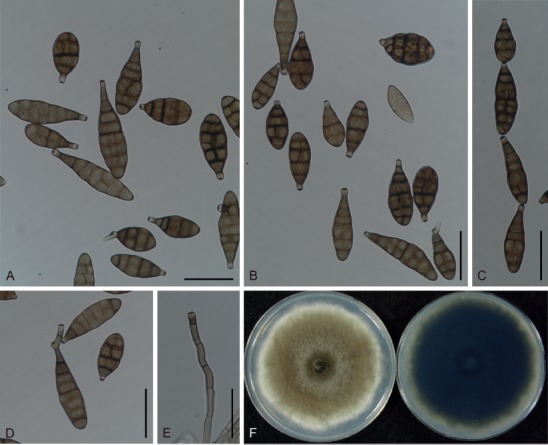
Morphological features of Japanese isolates of Alternaria gaisen f. sp. fragariae (MAFF 242310) on potato-carrot agar medium. A–D. Conidia. E. Conidiophore. F. Culture on potato-dextrose agar medium (left = surface, right = reverse). Scale bars (A–E) = 25 µm.
Synonyms: Alternaria gaisen Nagano, J. Jpn. Hort. Soc. (Nihon Engei Zasshi) 32 (3): 16. 1920, nom. inval. (provisional name; ICN Art. 36.1).
Alternaria gaisen Nagano, in Hara, Jitsuyo Sakumotsu Byorigaku: 263. 1925.
Alternaria gaisen Nagano ex Hara, Sakumotsu Byorigaku, Edn 4: 263. 1928, in Woudenberg et al., Stud. Mycol. 82: 15. 2015, nom. superfl.
Alternaria kikuchiana S. Tanaka, Mem. Coll. Agric. Kyoto Imp. Univ. 28 (Phytopathol. Ser. 6): 27. 1933.
Typus: Japan, Nara Prefecture, on leaves of Pyrus pyrifolia var. culta ‘Nijisseiki’ (details unknown; not preserved). Lectotype, Nagano K., J. Jpn. Hort. Soc. (Nihon Engei Zasshi) 32 (3): 17, figures (iconotype, selected by Simmons 2007). Epitype, Japan, Tottori Prefecture, on P. pyrifolia var. culta, Jul. 1990, E.G. Simmons, dried culture specimen CBS H-22842 (designated in Nishikawa & Nakashima 2019), culture ex-epitype CBS 118488 = EGS 90.0391.
Additional materials examined: Japan, Iwate Prefecture, Morioka, on leaves of Fragaria × ananassa ‘Morioka-16’, 1975, Y. Watanabe, living cultures M-11 (MAFF 731001), M-14 (MAFF 731002), M-15 (MAFF 731003), M-17 (MAFF 731004), M-20 (MAFF 731005), M-22 (MAFF 731006), M-23 (MAFF 731007); Tottori Prefecture, Tohaku, Hokuei, Horticultural Research Center, on leaves of P. pyrifolia var. culta ‘Nijisseiki’, Jul. 1999, F. Yasuda, living cultures 9901A (MUCC 2151), 9903A (MUCC 2152), 9904C (MUCC 2153); Hokkaido, Esashi, on leaves of Fragaria × ananassa ‘HS-138’, Aug. 2007, T. Misawa, dried culture specimen MUMH 11698, living culture E-11 (MAFF 242310 = MUCC 1609); Hokkaido, Hokuto, on leaves of Fragaria × ananassa ‘HS-138’, 22 May 2008, T. Misawa, MUMH 11681 (inoculated leaves with MAFF 242310).
Morphological characters on PCA medium: Previously reported in Nishikawa & Nakashima (2019).
Colony characteristics on PDA after 7 d at 25 °C: Fast-growing, reaching an average of 82.1 ± 0.7 mm diam; aerial hypha cottony, grayish green to dark green, with white margins; reverse center dark green to black; sporulation abundant; diffusible pigment absent.
Sexual morph: Not observed.
Natural hosts: Fragaria × ananassa ‘Morioka-16’, P. pyrifolia var. culta ‘Nijisseiki’, and their related cultivars (Rosaceae).
Symptoms: Leaf spots on strawberries are circular, 3–12 mm diam, brown to black with a reddish-brown border and a yellowish halo that is grayish brown at the center, which may be extend by the fungal toxin.
Host range: It is known as a host-selective toxin producer, which is pathogenic to strawberry cultivar ‘Morioka-16’ and Japanese pear cultivar ‘Nijisseiki’, and a few of their related lines (Hayashi et al. 1992).
Distribution: Limited to Japan (Nagano 1920, Watanabe & Umekawa 1977), New Zealand (Dingley 1970), Korea (Cho & Moon 1980), and Italy (Wada et al. 1996).
Distinctive features: Conidia form in short, unbranched chains. It is pathogenic to only a few cultivars of strawberry (cv. Morioka-16) and Japanese pear (cv. Nijisseiki) owing to its production of AF-toxin. Phylogenetically, it is clearly distinguishable from the other species of this section via gapdh, rpb2, Alt a 1 and endoPG sequences (Figs 1, 3).
Notes: Within the species, A. gaisen includes two formae speciales, A. gaisen f. sp. pyri producing the AK-toxin (toxic to Japanese pear), and f. sp. fragariae producing the AF-toxin (toxic to strawberry) (Nishikawa & Nakashima 2019).
Alternaria iridicola (Ellis & Everh.) J.A. Elliott, Am. J. Bot. 4: 450. 1917. Figs 22, 23.
Fig. 22.
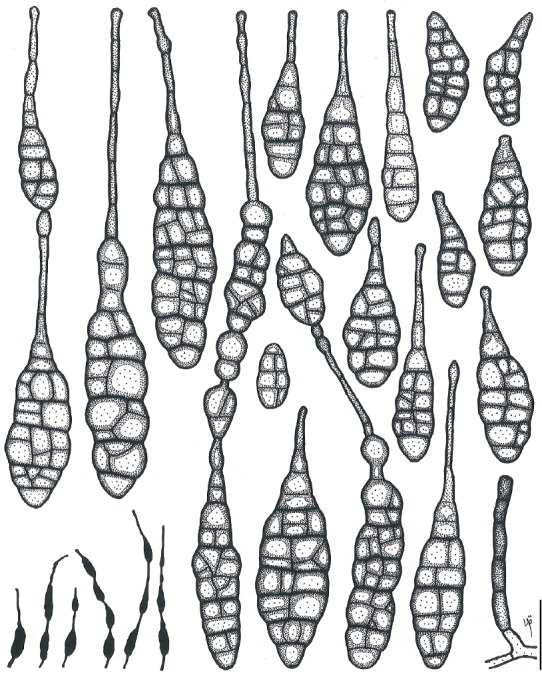
Illustrations of Alternaria iridicola (MAFF 246771). Morphology of conidia and conidiophores, and sporulation patterns (opaque) on potato-carrot agar medium. Scale bar = 25 µm.
Fig. 23.

Morphological features of Japanese isolates of Alternaria iridicola (MAFF 246890) on potato-carrot agar medium. A–J. Conidia. K. Conidiophores. L. Culture on potato-dextrose agar medium (left = surface, right = reverse). M. Dried culture specimen ex MAFF 246890 (epitype: TNS-F-85452). N. Specimens of diseased leaves of Iris japonica (isoepitype: MUMH 11687). Scale bars (A–K) = 25 µm.
Basionym: Macrosporium iridicolum Ellis & Everh., Proc. Acad. Nat. Sci. Philad. 46(3): 382. 1894.
Typus: USA, Idaho, Moscow, on Iris missouriensis, 27 May 1894, Henderson, holotype NY 2640. Epitype designated here, Japan, Kanagawa Prefecture, Kamakura, on leaves of Iris japonica, 17 Apr. 2013, H. Horie, TNS-F-85452 (dried culture specimen of MAFF 246890) [MBT385027], isoepitypes MUMH 11687and MUMH 11739, culture ex-epitype MAFF 246890 = MUCC 2149.
Additional materials examined: Japan, Tokyo, Kodaira, on I. japonica, 2010, H. Horie, living culture MUCC 2148; Shizuoka Prefecture, Fukuroi, Ugari, on I. japonica, 24 Mar. 2018, J. Nishikawa, MUMH 11690 and MUMH 11697, living culture MAFF 246771 = MUCC 2501.
Morphological characters on PCA medium: Conidiophores solitary to fascicular, subcylindrical, unbranched, straight or sometimes geniculate, thin, 23–128 × 4–6 μm. Conidia either solitary, or in short chains of 3–4 conidia without lateral branches. Conidia vary in size and appear as distorted, ovoid, ellipsoid to broadly obclavate, or sometimes beakless small oval, pale brown to yellowish brown, 28–311 × 7–38 µm in total. Conidial bodies 21–127 × 7–38 μm, with 2–16 transverse and 0–11 longitudinal septa, constricted at some transverse septa, commonly with distosepta-like internal wall structure. Secondary conidiophores appear at the apical end of conidia, short to long, usually unbranched, often with swollen cells inserted, 6–200 × 2–7 μm. Conidia of ex-epitype culture MAFF 246890 on PCA medium 25–114 × 11–38 μm, with 2–16 transverse and 1–11 longitudinal septa; secondary conidiophores 6–200 × 2–7 μm.
Colony characteristics on PDA after 7 d at 25 °C: Moderate-growing, reaching an average of 67.2 ± 3 mm diam, variable among strains; aerial hypha cottony, gray to pale grayish-green, with white margins; reverse center grayish green to dark green; sporulation sparse; diffusible pigment absent.
Sexual morph: Not observed.
Natural hosts: Iris (including Belamcanda chinensis) and Gladiolus (Iridaceae) (Yu 2001).
Symptoms: Leaf spots on I. japonica appear grayish brown surrounded by a yellowish halo. They are distinctly circular, and become enlarged and confluent, with caespituli abundantly formed at the center, and measuring 5–40 mm diam.
Experimental host range: Restricted to Iris excluding I. ensata, and weakly or non-pathogenic to the other examined plants including Gladiolus and Allium (Table 8). Similar results on Allium were also obtained by Elliot (1917).
Distribution: China, Japan, Korea, and USA (Shimazaki 1930, Tohyama 1993, Yu 2001, Zhang 2003, Simmons 2007).
Distinctive features: Notably large spores among sect. Alternaria commonly arising from narrow conidiophores. Conidia often have a long secondary conidiophore with cellular swellings. The pathogenicity of this species is restricted to certain Iris spp. with some species-selectivity. Phylogenetically, it is clearly distinguishable from the other species of this section in gapdh, rpb2, tef1, Alt a 1, and endoPG sequences (Figs 1, 3).
Notes: Based on conidial morphology of the holotype material, Simmons (2007) suggested that previous morphological descriptions of the species by Elliott (1917), Joly (1964), and Zhang (2003), which described conidial chains and long beaked conidia, could be misidentifications of other A. tenuissima-like species. However, conidial morphology obtained from fresh Japanese materials was identical with those in the original descriptions (Ellis & Everhart 1894, Elliott 1917), and also contained the unrepresentative beakless conidia illustrated by Simmons (2007) (Fig. 23F–J). Because diagnostic conidia of the species are scarcely present on the holotype, and no reliable living isolates exist in public culture collections, one of the Japanese specimens, TNS-F-85452, was newly designated as epitype in the present study. Although Yu (2001) has added Gladiolus as a natural host in Korea, without photos and details, no distinct symptoms and sporulation were observed on the inoculated leaves in this study.
Section Brassicicola D.P. Lawr. et al., Mycologia 105: 541. 2013.
Five species were recognized in this section based on the multi-locus phylogeny reported by Woudenberg et al. (2013), excluding A. japonica. This section was morphologically characterized by ellipsoidal to ovoid conidia formed in long chains, with apical conidiogenous cells and no or a few longitudinal septa (emending the description of sect. Brassicicola sensu Lawrence et al. 2013). However, neither the morphological nor the pathological differences among these species have been defined.
Alternaria brassicicola (Schwein.) Wiltshire, Mycol. Pap. 20: 8. 1947. Figs 24, 25.
Fig. 24.
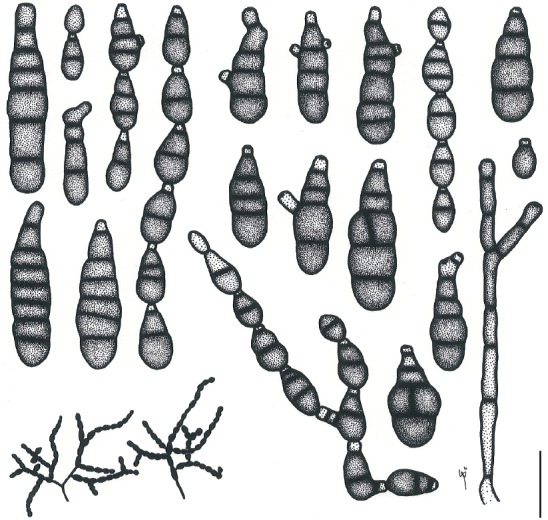
Illustrations of Alternaria brassicicola (MAFF 246772). Morphology of conidia and conidiophores, and sporulation patterns (opaque) on potato-carrot agar medium. Scale bar = 25 µm.
Fig. 25.

Morphological features of Japanese isolates of Alternaria brassicicola on potato-carrot agar medium. A–D. Conidia and conidiophores (MAFF 246772). E. Conidia ex MAFF 246773. F. Culture on potato-dextrose agar medium (MAFF 246772; left = surface, right = reverse). G. Natural symptoms on Brassica oleracea var. sabellica. Scale bars (A–E) = 25 µm.
Basionym: Helminthosporium brassicicola Schwein. (as ‘brassicola’), Trans. Amer. Philos. Soc. 4(2): 279. 1832.
Synonyms: Sporidesmium septorioides Westend., Bull. Acad. Roy. Sci. Belgique., Cl. Sci., Sér. 2, 21: 236. 1854.
Alternaria septorioides (Westend.) E.G. Simmons, CBS Biodiversity Ser. (Utrecht) 6: 570. 2007.
Sporidesmium exitiosum f. alternarioides J.G. Kühn, Hedwigia 1: 91. 1855.
Polydesmus exitiosus f. alternarioides (J.G. Kühn) J.G. Kühn, Hedwigia 1: 165. 1858.
Sporidesmium exitiosum f. luxuriosum J.G. Kühn, Hedwigia 1: 91. 1855.
Polydesmus exitiosus f. luxuriosum (J.G. Kühn) J.G. Kühn, Die Krankheiten der Kulturgewächse, ihre Ursachen und Verbreitung: 165. 1858.
Macrosporium circinans Berk. & M.A. Curtis, in Curtis, N. Carol. Geol. Nat. Hist. Surv. 3: 128. 1867, nom. nud.
Macrosporium cheiranthi var. circinans Berk. & M.A. Curtis, in Berkeley, Grevillea 3 (27): 105. 1875.
Macrosporium commune var. circinans (Berk. & M.A. Curtis) Sacc., Syll. Fung. 4: 524. 1886.
Alternaria circinans (Berk. & M.A. Curtis) P.C. Bolle, Meded. Phytopath. Labor. ‘WCS’ 7: 26. 1924.
Alternaria brassicae var. minor Sacc., Michelia 2(6): 172. 1880.
Helminthosporium brassicae Henn., Hedwigia 41: 117. 1902.
Alternaria oleracea Milbr., Bot. Gaz. 74(3): 321. 1922.
Alternaria brassicae var. microspora J.A. Elliott, in Neergaard, Danish species of Alternaria and Stemphylium: 129. 1945.
Alternaria mimicula E.G. Simmons, Mycotaxon 55: 129. 1995.
Alternaria solidaccana E.G. Simmons, CBS Biodiversity Ser. (Utrecht) 6: 572. 2007.
Typus: on petioles of Brassica oleracea var. capitata (details unknown; not preserved). Lectotype EGS 05.167, slide glass preparation “Helminthosporium brassicola S [sic] / valde memoria Beth. in cella nostra”, in the Schweinitz herbarium at PH (designated in Simmons 1995a).
Ex-type culture: Unknown.
Additional materials examined: Japan, Shizuoka Prefecture, Kakegawa, on leaves of Brassica oleracea var. sabellica, 13 Mar. 2003, J. Nishikawa, MUMH 11667, living culture MAFF 246772 = MUCC 1694; Tokyo, Setagaya, from seeds of Spinacia oleracea, 13 Feb. 2002, J. Nishikawa, living culture MAFF 246773; Shizuoka Prefecture, Kakegawa, on leaves of Brassica rapa subsp. pekinensis, 5 Nov. 2008, J. Nishikawa, MUMH 11683, living culture MUCC 1612 = AC56; Tokyo, Setagaya, from seeds of Raphanus sativus, Jul. 2000, J. Nishikawa, living culture MUCC 1619 = AC70 and AC71; ibid., from seeds of B. oleracea var. italica, 2001, J. Nishikawa, living culture AC72.
Morphological characters on PCA medium: Conidiophores solitary, often branched, straight, intermediately broad, 6–60 × 3–6 μm. Conidia in chains 7–10 more conidia with frequent lateral branches, resulted in 30 more conidial units from one conidiophore. Conidial bodies, ovoid to ellipsoid, subcylindrical at maturity, brown to dark brown, 8–60 × 6–16 μm, with 0–8 transverse and few longitudinal thicken septa, mostly smooth to occasionally roughened; conidiogenous cell at terminal conidia (secondary conidiophores) short, mostly single-celled. onidia on lesions also similar but somewhat larger than those on PCA.
Colony characteristics on PDA after 7 d at 25 °C: Moderate-growing, reaching an average of 59.5 ± 1.4 mm diam; aerial hypha sparse, dark green to black; reverse center gray; sporulation abundant; diffusible pigment absent.
Sexual morph: Not observed.
Natural hosts: Brassicaceae. Simmons (2007) and Farr & Rossman (2018) also recorded on Digitalis (Plantaginaceae) and non-brassicaceous plants.
Symptoms: Leaf spots on B. oleracea, gray to brown, circular to zonate, 8–12 mm diam, enlarged and confluent; head rot (pin rot) of broccoli and cauliflower, water-soaked to discolored on buds. Caespituli were frequently observed on lesions.
Experimental host range: Strongly pathogenic to Brassicaceae including Diplotaxis, Matthiola, Iberis, and Nasturtium; weakly pathogenic Lobularia, to Eutrema and Aubrieta; non-pathogenic to Capsella and non-Brassicaceae plants (Table 5).
Distribution: Worldwide, including Asia (Bangladesh, Brunei, China, India, Indonesia, Japan, Korea, Malaysia, Mongolia, Myanmar, Nepal, Pakistan, Saudi Arabia, Sri Lanka, Taiwan, Thailand, and Uzbekistan), Europe (Bulgaria, Cyprus, Denmark, France, Greece, Italy, Netherlands, Poland, Romania, Russia, Turkey, and UK), North and Latin America (Barbados, Brazil, Canada, Chile, Cuba, Jamaica, Panama, USA, and Venezuela), Africa (Ethiopia, Ghana, Guinea, Libya, Malawi, Mauritius, Nigeria, Rhodesia, Sierra Leone, South Africa, Sudan, Tanzania, Tunisia, Uganda, Zambia, and Zimbabwe), and the Pacific (Australia, Cook Islands, New Caledonia, New Zealand, Papua New Guinea, Samoa, and Tonga) (Yoshii 1941, Ellis 1971, Yu 2001, Zhang 2003, Farr & Rossman 2018).
Distinctive features: Small conidia form in long chains, frequently with lateral branches and rarely with longitudinal septa. Basal conidia form in subcylindrical to oblong. This species was widely pathogenic to Brassicaceae, but non- or weakly to Eutrema, Aubrieta and Capsella. It is phylogenetically recognizable via its ITS (Fig. 2), gapdh, tef1, rpb2, Alt a 1, and act sequences (data not shown).
Notes: Alternaria septorioides, A. mimicula, and A. solidaccana were synonymized in the present study based on their phylogeny using each ex-type isolate, morphological similarity, restricted host range within Brassicaceae plants, and the ubiquitousness and saprophytic habit of the species. Likewise, A. conoidea is a possible synonym of the species (CBS 132.89 is not authentic), and, therefore, sect. Brassicicola may be a monotypic lineage as supported by the ITS phylogeny (Fig. 2).
Section Crivellia (Shoemaker & Inderb.) Woudenb. & Crous, Stud. Mycol. 75: 189. 2013.
Basionym: Crivellia Shoemaker & Inderb., Canad. J. Bot. 84: 1308. 2006.
Two species are assigned in this section, which is morphologically characterized by cylindrical conidia forming in chains of geniculate conidiophores and microsclerotial formation (Woudenberg et al. 2013). Both of the species are known as pathogenic to Papaver spp., having a sexual morph formerly known as Crivellia (Inderbitzin et al. 2006).
Alternaria penicillata (Corda) Woudenb. & Crous, Stud. Mycol. 75: 190. 2013. Fig. 26.
Fig. 26.
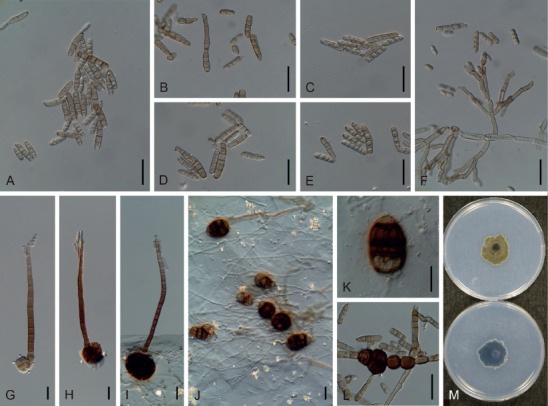
Morphological features of Japanese isolates of Alternaria penicillata (MUCC 1657) on V8 juice agar medium. A–E. Conidia. F. Conidiophore arising from aerial mycelium (microconidiophores). G–I. Conidiophores arising from stroma (macroconidiophores). J, K. Globose knotted cells (microsclerotia). L. Intercalary chlamydospore-like cells. M. Culture on potato-dextrose agar medium (upper = surface, lower = reverse). Scale bars (A–L) = 25 µm.
Basionym: Brachycladium penicillatum Corda, Icon. Fung. 2: 14. 1838.
Synonyms: Dendryphion penicillatum (Corda) Fr., Summa Veg. Scand., Sect. Post. (Stockholm): 504. 1849.
Cucurbitaria papaveracea De Not., Sferiacei Italici: 62. 1863.
Pleospora papaveracea (De Not.) Sacc., Syll. Fung. 2: 243. 1883.
Crivellia papaveracea (De Not.) Shoemaker & Inderb., Canad. J. Bot. 84: 1308. 2006.
Dendryphion penicillatum var. sclerotiale M.-E. Meffert, Z. ParasitKde 14 (5): 462. 1950, nom. nud. (ICN Art. 39.1).
Typus: Czech, Praha, on Papaver sp., 6 Dec. 1837, holotype DAOM 49356 in PR. Epitype, Austria, Vienna, on stems of Papaver rhoeas, DAOM 230456 (P354) (designated in Inderbitzin et al. 2006), culture ex-epitype P354.8 = CBS 116608.
Additional material examined: Japan, Tokyo, Tachikawa, on leaves of Papaver nudicaule, 13 Jun. 2005, Y. Makizumi, living culture MUCC 1657.
Morphological characters on V8 medium: Globose knotted cells (microsclerotia), brown to dark reddish brown, 30–65 × 25–45 μm. Conidiophores arising from stroma (macroconidiophores), brown to reddish brown, straight and long, 128–225 × 8–11 μm, with short and sub-hyaline conidiogenous cells at the apex. Conidiophores arising from aerial mycelia (microconidiophores), geniculate with sympodial proliferation, branches often arboroid, short and narrow, 19–98 × 3–6 μm. Conidia commonly in short chains of 2–5, ellipsoid to cylindrical, subhyaline to pale brown, 8–38 × 4–6 μm in total, with 0–6 transverse and no longitudinal septa, remaining as distosepta-like, smooth structures. Intercalary chlamydospore-like cells, dark brown and roughened, in knots or chains, 11–29 × 10–24 μm.
Colony characteristics on PDA after 7 d at 25 °C: Slow-growing, reaching 28.7 ± 4.5 mm diam; aerial hypha cottony, olive brown, with white margins; reverse center dark green to black; sporulation abundant; diffusible pigment absent.
Sexual morph: Known formerly as the genus Crivellia, but not observed in the present study.
Natural host: Papaver (Papaveraceae).
Distribution: Worldwide, including Asia (Afghanistan, India, Iran, Japan, and Korea), Europe (Austria, Azerbaijan, Czech, Germany, Hungary, Netherlands, Poland, Romania, Russia, Spain, Sweden, Switzerland, Turkey, UK, and Ukraine), North and Latin America (USA, Colombia, and Venezuela), Australia, and South Africa (Hirayama & Imura 1941, Richardson 1990, Farr et al. 2000, Inderbitzin et al. 2006, Hyun et al. 2012, Gasich et al. 2013, Woudenberg et al. 2013, Farr & Rossman 2018).
Distinctive features: Subhyaline to pale brown conidia appearing in chains, and macroconidiophores, microconidiophores, and microsclerotia are also present. It is phylogenetically distinguishable via its ITS (Fig. 2), gapdh, tef1, rpb2, Alt a 1, and act sequences (data not shown).
Section Eureka Woudenb. & Crous, Stud. Mycol. 75: 193. 2013.
Six species are assigned to this section, which are characterized by simple, short, and broad conidiophores, and ellipsoidal to cylindrical conidia that are either solitary or appear in short chains (Woudenberg et al. 2013). As to A. eureka, the type species of this section, a sexual morph has been reported (Simmons 1986).
Alternaria cumini E.G. Simmons, CBS Biodiversity Ser. (Utrecht) 6: 664. 2007. Figs 27, 28.
Fig. 27.
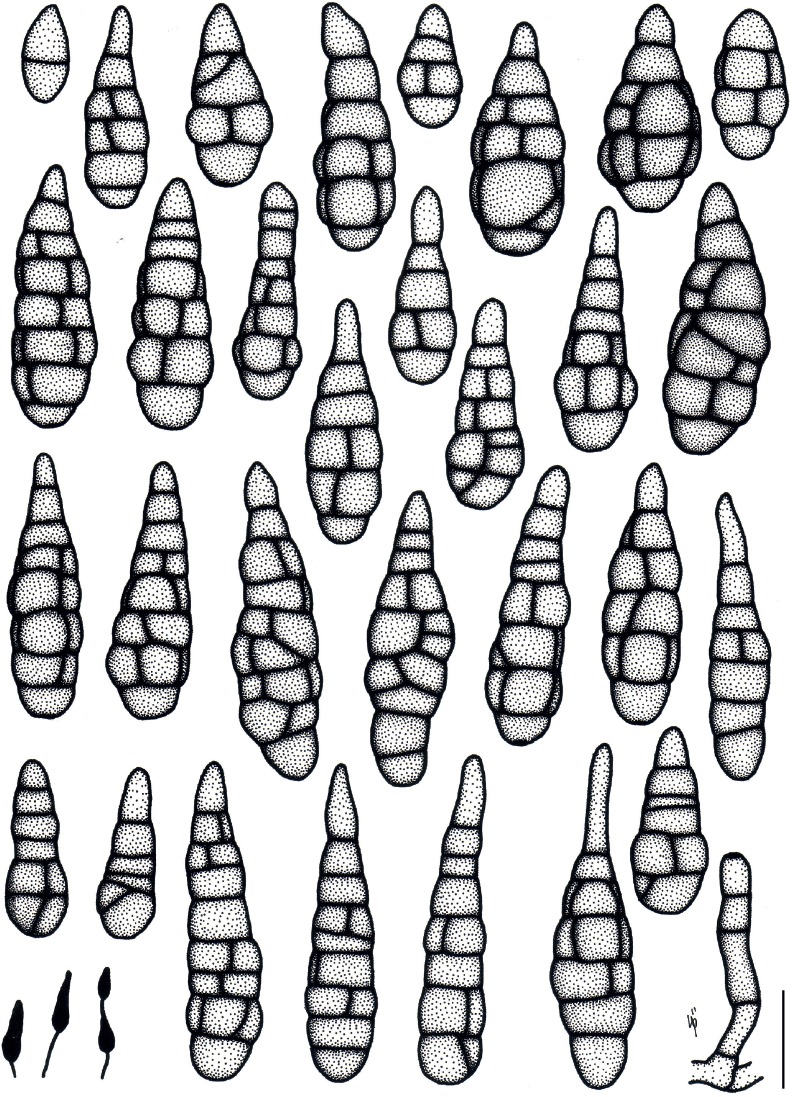
Illustrations of Alternaria cumini (MAFF 246774). Morphology of conidia and conidiophores, and sporulation patterns (opaque) on V8 juice agar medium. Scale bar = 25 µm.
Fig. 28.

Morphological features of Japanese isolates of Alternaria cumini (MAFF 246774). A–C. Conidia and conidiophores on V8 juice agar medium. D. Culture on potato-dextrose agar medium (left = surface, right = reverse). E. Natural symptoms (damping-off of seedlings and leaf blight) on seedling of Cuminum. Scale bars (A–C) = 25 µm.
Typus: India, Gujarat, Karli, on Cuminum cyminum, Jan. 1954, M.K. Patel, holotype BPI 877406 (dried culture specimen ex CBS 121329), culture ex-holotype CBS 121329 = EGS 04.1581.
Additional materials examined: Japan, Shizuoka Prefecture, Kakegawa, on leaves of C. cyminum, 17 May 2012, J. Nishikawa, living culture MAFF 246774; ibid., 18 May 2013, J. Nishikawa, living culture AC115.
Morphological characters on V8 medium: Conidiophores erect, short and narrow, 18–60 × 5–7 µm. Conidia solitary, rarely in chains, brown to dark brown, obclavate to long ellipsoid, subcylindrical, smooth, 23–76 × 8–26 µm, with 1–9 transverse and 0–5 longitudinal septa, slightly constricted at each transverse segment, beakless, but most with a conical cell at the apex. Morphology on PCA medium similar to that on V8 medium: conidiophores 25–93 × 5–6 µm; conidia 24–61 × 13–25 µm, with 2–7 transverse and 1–5 longitudinal septa.
Colony characteristics on PDA after 7 d at 25 °C: Moderate-growing, reaching an average of 62.7 ± 1.1 mm diam; aerial hypha cottony, pale gray to grayish green, with white to pale gray margins; reverse center brownish green to dark green; sporulation sparse; diffusible pigment absent.
Sexual morph: Not observed.
Natural hosts: Cuminum (Apiaceae).
Symptoms: Damping-off of Cuminum seedlings, and experimentally-caused leaf blight.
Experimental host range: Strongly pathogenic to Cuminum; weakly pathogenic or opportunistic to Anthriscus and Petroselinum; but non-pathogenic to other Apiaceae plants, including Daucus (Table 6).
Distribution: Japan and India (Simmons 2007).
Distinctive features: Beakless, solitary conidia with a conical apical cell. Conidiophores are long and broad. Host range of the species is highly selective to Cuminum, and it is phylogenetically recognizable via its ITS, gapdh, tef1, rpb2, Alt a 1, and act sequences based on the DDBJ BLASTn results (data not shown).
Note: This is the first record other than the type locality.
Section Gypsophilae D.P. Lawr. et al., Mycologia 105: 541. 2013.
Eight species were assigned to this section (Woudenberg et al. 2013). This section is morphologically characterized by large conidia formed in short chains, with a short, blunt-tapered false beak and multiple septa (Lawrence et al. 2013). In Russia, Gannibal (2019) proposed an additional species on Dianthus, A. kamtschatica, based on multi-locus phylogeny using gapdh, calmodulin, and Alt a 1 sequences. All the recognized species in this section occur on Caryophyllaceae.
Alternaria nobilis (Vize) E.G. Simmons, Mycotaxon 82: 7. 2002. Fig. 29.
Fig. 29.

Morphological features of Japanese isolates of Alternaria nobilis (AC1) on V8 juice agar medium. A–E. Conidia. F. Conidiophores. G. Culture on potato-dextrose agar medium (upper = surface, lower = reverse). H. Natural symptoms on Dianthus. Scale bars (A–F) = 25 µm.
Basionym: Macrosporium nobile Vize, in Cooke, Grevillea 5(35): 119. 1877.
Synonyms: Alternaria dianthi F. Stevens & J.G. Hall, Bot. Gaz. 47(5): 413. 1909.
Macrosporium dianthi F. Stevens & J.G. Hall, in Bewley, Diseases of Glasshouse Plants: 106. 1923, nom. illeg. (later homonym; ICN Art. 53.1). non Macrosporium dianthi J.V. Almeida & Sousa da Câmara, Revista Agron. 1: 59. 1903.
Typus: UK, Forden, on stems and leaves of Dianthus caryophyllus, 1877, J.E. Vize (holotype not specified). Lectotype, K, EGS 11.014 (designated in Simmons 2002), isolectotype (probable) IMI 57062 (J.E. Vize, Micro-Fungi Britannici no. 63, Macrosporium nobile Vize 1878).
Ex-type culture: Unknown.
Additional materials examined: Japan, Shizuoka Prefecture, Kakegawa, on leaves of Dianthus barbatus, 5 Jun. 2003, J. Nishikawa, living culture AC1; Miyagi Prefecture, Sendai, on leaves of D. caryophyllus, 12 Nov. 2002, Y. Makizumi, living culture AC25.
Morphological characters on V8 medium: Conidiophores erect, broad, pale brown to brown, unbranched, 16–60 × 5–9 µm. Conidia solitary to commonly in chains of 2–5 conidia, without or rarely with lateral branches, yellowish brown to brown, oblong to long obclavate, with a blunt-tapered false beak, almost straight, not swollen, smooth, clearly constricted at each transverse septa, 14–141 µm in total length. Conidial bodies 14–100 × 6–30 µm, with up to 16 transverse septa and 13 longitudinal septa. False beaks not filamentous, usually unbranched and short, consist of 2–3 cells, 3–52 × 3–6 µm.
Colony characteristics on PDA after 7 d at 25 °C: Slow-growing, reaching an average of 39.2 ± 1.4 mm diam; aerial hypha cottony, dense, grayish green, with white margins; reverse center dark green to black; sporulation sparse; diffusible pigment absent.
Sexual morph: Sexual form not observed.
Natural hosts: Primarily Dianthus, Gypsophila, Lychnis, and Saponaria (Caryophyllaceae), and also recorded on Calendula (Asteraceae), Hibiscus (Malvaceae), Jasminum (Oleaceae), Lolium (Poaceae), and Sesamum (Pedaliaceae) (Rao 1969, Richardson 1990, Garibaldi et al. 2013, Farr & Rossman 2018).
Symptom: Leaf spots on Dianthus are circular to zonate, grayish brown to brown, and become enlarged and confluent, reaching 4–7 mm with a necrotic eye at the center, and sometimes with a chlorotic halo around the primary lesion.
Distribution: Worldwide, including Asia (China, India, Japan, Korea, Malaysia, Myanmar, Pakistan, and Thailand), Europe (Armenia, Austria, Bulgaria, Croatia, Cyprus, Denmark, France, Germany, Greece, Italy, Latvia, Poland, Romania, Russia, Spain, Sweden, Turkey, and UK), North and Latin America (Argentina, Brazil, Canada, Jamaica, Mexico, Puerto Rico, Uruguay, USA, Venezuela, and Virgin Islands), Africa (Malawi, Morocco, Mozambique, South Africa, Tanzania, Zambia, and Zimbabwe), and the Pacific (Australia and New Zealand) (Imai 1914, Rao 1969, Ellis 1971, Richardson 1990, Cho et al. 2001, Yu 2001, Garibaldi et al. 2013, Woudenberg et al. 2013, Farr & Rossman 2018).
Distinctive features: Large conidia, solitary to in short chain with a blunt-tapered false beak and multiple septa. Conidiophores long and broad. This species is pathogenic to Dianthus.
Notes: Morphological characteristics of the examined Japanese isolates were identical to those of A. nobilis described in Simmons (2002). However, these were also similar to those of the other members in sect. Gypsophilae: shorter than the conidial length of A. saponariae; larger than the conidial width of A. ellipsoidea and A. kamtschatica according to the criteria in Simmons (2002) and Gannibal (2019). The multi-locus phylogeny conducted in this study showed that examined isolates of this section were divided into five subclades, and Japanese isolates formed a distinct subclade (Fig. 1), while ITS (Fig. 2) as well as tef1 and act phylogeny proposed that Japanese isolates are identical with the representative isolate of A. nobilis (data not shown). It is still unclear which trait can practically determine species boundaries among them, and therefore, we provisionally identified Japanese isolates as A. nobilis based on their morphological similarity and original source host, Dianthus.
Section Japonicae Woudenb. & Crous, Stud. Mycol. 75: 197. 2013.
Woudenberg et al. (2013) assigned two species to this section, which is morphologically characterized by short- to long-ovoidal conidia formed in short chains, with constrictions at the septa. However, neither the morphological nor the pathological differences between these two species have been well defined.
Alternaria japonica Yoshii, J. Pl. Protect. (Tokyo) 28: 17. 1941. Figs 30, 31.
Fig. 30.
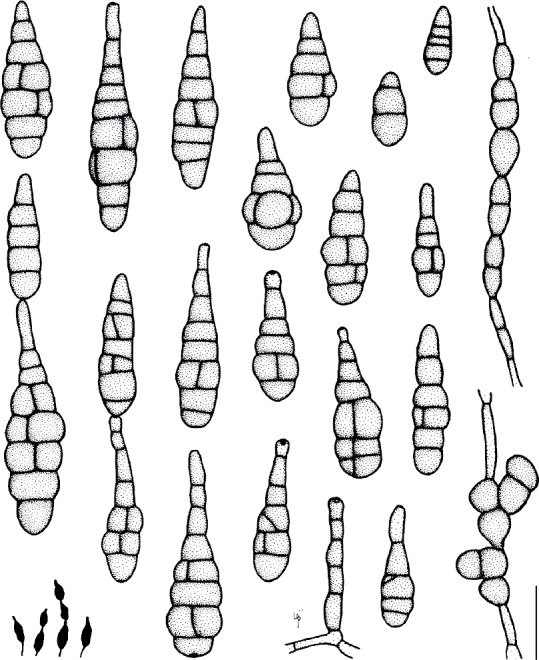
Illustrations of Alternaria japonica (MAFF 246775). Morphology of conidia and conidiophores, and sporulation patterns (opaque) on potato-carrot agar medium. Scale bar = 25 µm.
Fig. 31.
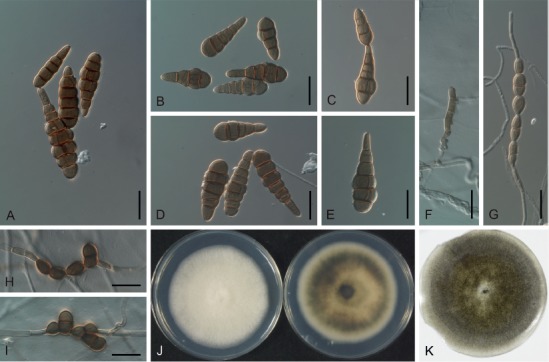
Morphological features of Japanese isolates of Alternaria japonica (MAFF 246775) on potato-carrot agar medium. A–E. Conidia. F. Conidiophore. G–I. Chlamydospores. J. Culture on potato-dextrose agar medium (left = surface, right = reverse). K. Dried culture specimen ex MAFF 246775 (epitype: TNS-F-85453). Scale bars (A–I) = 25 µm.
Synonyms: Alternaria brassicae (Berk.) Sacc. var. macrospora [non Sacc.] sensu Yoshii, Bult. Sci. Fak. Terk. Kjusu Imp. Univ. 5 (3): 224. 1933.
Alternaria raphani J.W. Groves & Skolko, Canad. J. Res., Sect. C 22 (5): 227. 1944.
Alternaria matthiolae Neerg., Danish species of Alternaria and Stemphylium: 184. 1945.
Alternaria nepalensis E.G. Simmons, CBS Biodiversity Ser. (Utrecht) 6: 480. 2007.
Typus: Japan, on leaves of Brassica rapa and Raphanus sativus (details unknown, not specified, not preserved). Lectotype, IMI 876 (designated in Tohyama & Tsuda 1990; the same specimen was designated as a neotype in Simmons 1995a). Epitype designated here, Japan, Tokyo, Setagaya, from seeds of R. sativus, 24 Jul. 2000, J. Nishikawa, TNS-F-85453 (dried culture specimen of MAFF 246775) (MBT385028), isoepitype MUMH 11696, culture ex-epitype MAFF 246775 = MUCC 1622.
Additional materials examined: Japan, Tokyo, Setagaya, from seeds of R. sativus, 24 Jul. 2000, J. Nishikawa, living cultures AC73; Shizuoka Prefecture, Kakegawa, on buds of Brassica oleracea var. italica, 7 Jun. 2010, K. Takebayashi, living culture AC96; ibid., on stem of B. oleracea var. italica, 7 Jun. 2010, K. Takebayashi, living culture AC97.
Morphological characters on PCA medium: Conidiophores solitary, short and narrow, 18–80 × 4–6 µm. Conidia of ex-epitype solitary or in short chains of 1–2, without lateral branches, 20–84 µm in total length. Conidial bodies ovoid to obclavate, ellipsoid, pale brown to brown and smooth, 20–68 × 8–25 µm, with 2–7 transverse septa and 0–4 longitudinal septa, constricted at some transverse septa. Secondary conidiophores (false beaks) usually short, 1–3 celled, unbranched, 5–20 × 4–10 µm. Intercalary chlamydospores frequently both in air and submerged in agar substrate, either as single spores or in knots or chains, brown to dark brown, 10–21 × 8–16 µm.
Colony characteristics on PDA after 7 d at 25 °C: Moderate-growing, reaching an average of 59.1 ± 2.9 mm diam, variable among strains; aerial hypha cottony, white or pale gray to grayish green, with white margins; reverse center dark green to black; sporulation sparse; diffusible pigment absent.
Sexual morph: Not observed.
Natural hosts: Brassicaceae. There are also reported exceptions of Carya (Juglandaceae), Kalanchoe (Crassulaceae), Oryza (Poaceae), Sesamum (Pedaliaceae), and Vigna (Fabaceae) serving as hosts (Huang & Hanlin 1975, Farr & Rossman 2018).
Symptoms: Head rot and leaf spot like those caused by A. brassicicola, but caespituli appear sparsely on lesions.
Experimental host range: Strongly pathogenic to Brassicaceae, including Diplotaxis, Matthiola, and Nasturtium; weakly pathogenic or opportunistic to Lobularia, Eutrema, Iberis, and Aubrieta; almost non-pathogenic to Capsella and non-Brassicaceae plants (Table 5).
Distribution: Worldwide, including Asia (China, India, Japan, Korea, Myanmar, Nepal, Pakistan, Taiwan, and Saudi Arabia), Europe (Austria, Denmark, Finland, Germany, Greece, Italy, Netherlands, Poland, Russia, and Spain), North and Latin America (Barbados, Brazil, Canada, Cuba, and USA), Africa (Egypt, South Africa, Tunisia, and Zimbabwe), and the Pacific (Australia, New Caledonia, New Zealand, and Papua New Guinea) (Yoshii 1941, Rao 1969, Ellis 1971, Richardson 1990, Tohyama & Tsuda 1990, Jasalavich et al. 1995, Sharma & Tewari 1998, Yu 2001, Zhang 2003, Su et al. 2005, Simmons 2007, Gannibal & Gasich 2009, Ren et al. 2012, Bassimba et al. 2013, Woudenberg et al. 2013, Siciliano et al. 2017, Farr & Rossman 2018).
Distinctive features: Small conidia are either solitary or appear in short chains. Intercalary chlamydospores frequently form both in air and submerged in agar substrate. This species is widely pathogenic to Brassicaceae, but non- or weakly to Eutrema, Aubrieta and Capsella. It is phylogenetically recognizable via its ITS (Fig. 2), gapdh, tef1, rpb2, Alt a 1, and act sequences (data not shown).
Notes: This species is often confused under the names, A. raphani and A. matthiolae. However, A. japonica has nomenclatural priority to these two epithets. As for A. nepalensis described by Simmons (2007), it was clearly appropriate to synonymize it with A. japonica based on its conidial morphology, phylogenetic analysis, and its original source (from seeds of Brassica sp.). There are no ex-type living cultures and therefore we designated an epitype herein to provide reference for further molecular studies.
Section Panax D.P. Lawr. et al., Mycologia 105: 541. 2013.
Woudenberg et al. (2013) assigned five species to this section. This was morphologically characterized by small to large conidia with blunt-tapered false beaks and broad conidiophores. Two species, A. avenicola and A. photistica, have a sexual morph (Simmons 1986, 2007). Only one species, A. panax, is distributed in Japan, and was described in the present study.
Alternaria panax Whetzel, in Whetzel & Rosenbaum, Bull. U.S.D.A. Bur. Pl. Industr. 250: 11. 1912. Fig. 32.
Fig. 32.

Morphological features of Japanese isolates of Alternaria panax (MAFF 243161) on V8 juice agar medium. A–D. Conidia. E. Conidiophores. F. Culture on potato-dextrose agar medium (upper = surface, lower = reverse). G. Specimens of diseased leaves of Polyscias (MUMH 11686). Scale bars (A–E) = 25 µm.
Synonyms: Alternaria panax Whetzel, in Cowles, Science n. s. 29: 912. 1909, nom. nud.
Alternaria panacis Whetzel, in Saccardo, Syll. Fung. 25: 864. 1931, citing Rosenbaum & Zinnsmeister, J. Agric. Res. 5: 181. 1915, nom. illeg. (orthographic variant; ICN Art. 60.1).
Macrosporium araliae Dearn. & House, Circ. N.Y. St. Mus. 24: 58. 1940, nom. inval. (no Latin; ICN Art. 39.1).
Alternaria araliae H.C. Greene, Trans. Wisc. Acad. Sci. 42: 80. 1953. non Alternaria araliae sensu Deng et al., Mycol. Progr. 14(31): 4. 2015.
Alternaria actinophylla J.W. Mille, Fl. Dep. Agr., Div. Pl. Ind., Pl. Pathol. Circ. 80: 1969, nom. nud. (no Latin and type; ICN Art. 39.1, 40.1).
Typus: USA, New York, Fulton, on Panax quinquefolius, 15 Jun. 1909, H.H. Whetzel, BPI 446440 (isotype: fide Simmons 2007) ex CUP 4852; EGS 07.074.
Ex-type culture: Unknown.
Additional materials examined: Japan, Tokyo, Ogasawara (Bonin Is.), Chichijima, on leaves of Polyscias fruticosa, Jan. 2003, T. Ono, living culture PFAlt1-1 (MUCC 1692); ibid., on leaves of Polyscias guilfoylei, Apr. 2003, T. Ono, living culture PGAlt1 (AC19); Tokyo, Ogasawara (Bonin Is.), Hahajima, on leaves of P. fruticosa, 28 Oct. 2011, T. Sato, MUMH 11686, living cultures MAFF 243161 = MUCC 1625 and MAFF 243162 = MUCC 1626.
Morphological characters on V8 medium: Conidiophores broad, brown and unbranched, 55–145 × 7–10 µm. Conidia commonly in chains of 2–7, without or rarely with lateral branches, yellowish brown to brown, smooth, oblong to long obclavate, with a blunt-tapered false beak, mostly straight and laterally symmetrical but occasionally excessively swollen, often constricted at each transverse segment, 51–208 µm in total length. Conidial bodies 28–118 × 13–38 µm, with 4–13 transverse septa and up to 9 (often complicated) longitudinal septa; false beaks unbranched and not filamentous, 9–110 × 3–9 µm, pale brown to brown.
Colony characteristics on PDA after 7 d at 25 °C: Slow to moderate-growing, reaching an average of 46.5 ± 2.2 mm diam; aerial hypha cottony, white to pale gray; reverse center dark green to black; sporulation sparse; diffusible pigment absent or bright yellow to reddish orange.
Sexual morph: Not observed.
Natural hosts: Araliaceae (Acanthopanax, Aralia, Brassaia, Dendropanax, Echinopanax, Fatsia, Kalopanax, Meryta, Panax, Plerandra, Polyscias, Pseudopanax, Schefflera, and Tupidanthus) (Uchida et al. 1984, Yu 2001, Simmons 2007, Deng et al. 2010, 2013).
Symptoms: Leaf and petiole spots on Polyscias, appearing water-soaked to circular, brown, becoming enlarged and confluent, measuring 2–5 mm diam.
Distribution: Canada, China, Italy, Japan, Korea, New Zealand, and USA (Bokura 1915, Atilano 1983, Uchida et al. 1984, Yu 2001, Zhang 2003, Garibaldi et al. 2004, Ono 2004, Zhang et al. 2009, Deng et al. 2010, 2013, Woudenberg et al. 2013, Farr & Rossman 2018).
Distinctive features: Small and large conidia develop in short chains, with blunt-tapered false beaks. Colonies grown on PDA release either no pigment or a bright yellow to reddish orange pigment into the medium. This species is widely pathogenic to Araliaceae, and is phylogenetically recognizable via its ITS (Fig. 2), gapdh, tef1, rpb2, Alt a 1, and act sequences (data not shown).
Notes: Deng et al. (2015) split A. panax into three species, A. araliae, A. dendropanacis, and A. panax (as A. panacis, orthographic variant), based on their culture characteristics, phylogeny (Alt a 1, β-tubulin, tef1, gapdh, and rpb2), and hosts. However, among these, A. araliae sensu Deng et al. was unrelated to the type material of A. araliae H.C. Greene (BPI 445904). Japanese isolates examined in the present study could not accommodate the definitions of these three species, which focused on pigment production and conidial morphology, as established by Deng et al. (2015).
Section Porri D.P. Lawr. et al., Mycologia 105: 541. 2013.
This section is the largest and, morphologically, the most confusable section. It was morphologically characterized by large spores that were usually non-catenate with filamentous beaks and broad conidiophores, consisting of 63 species as defined by Woudenberg et al. (2014). Among these, only 12 species are distributed in Japan (NIAS Genebank database of plant diseases in Japan: https://www. gene.affrc.go.jp/databases-micro_pl_diseases_en.php), and five species are described in the present study.
Alternaria crassa (Sacc.) Rands, Phytopathology 7: 337. 1917. Fig. 33.
Fig. 33.

Morphological features of Japanese isolates of Alternaria crassa (MAFF 243056) on V8 juice agar medium. A–H. Conidia with colored beaks. I. Conidiophores. J. Culture on potato-dextrose agar medium (upper = surface, lower = reverse). K. Symptoms on specimens of Datura (MUMH 11688). Scale bars (A–I) = 25 µm.
Basionym: Cercospora crassa Sacc., Michelia 1(1): 88. 1877.
Synonyms: Cercospora daturae Peck, Rep. (Annual) New York State Mus. Nat. Hist. 35: 140. 1884.
Macrosporium cookei Sacc., Syll. Fung. 4: 530. 1886.
Macrosporium solani Cooke, Grevillea 12: 32. 1883. non M. solani Ellis & G. Martin, 1882.
Alternaria cookei (Sacc.) Bremer, Işmen, Karel & Özkan & M. Özkan, Istanbul Univ. Fak. Mecm., B 13: 42. 1948.
Macrosporium daturae Fautrey, in Lambottle & Fautrey, Rev. Mycol. (Toulouse) 16: 76. 1894.
Alternaria daturae (Fautrey) Bubák & Ranoj., in Kobát & Bubák, Fungi Imperf. Exsicc. 14: 694. 1911.
Alternaria capsici E.G. Simmons, Mycotaxon 75: 84. 2000.
Typus: on leaves of Datura stramonium (details unknown; two specimens in PAD). Lectotype, PAD, D. stramonium, S. [elva] ’76. 10. (designated in Simmons 2000). Epitype, Cyprus, Famagusta, on leaves of D. stramonium, Jan. 1936, R.M. Nattrass, CBS H-21744 (designated in Woudenberg et al. 2014), culture exepitype CBS 110.38.
Additional materials examined: Japan, Tokyo, Kodaira, on leaves of D. stramonium, Jul. 2000, J. Nishikawa, living culture MAFF 243056; ibid., on leaves of Datura fastuosa, 20 Oct. 2012, Ichinose et al., MUMH 11689, living culture MUCC 2502 (12-M0180); on leaves of Datura inoxia, 15 Sep. 2012, Ichinose et al., MUMH 11688, living culture MUCC 2503 (12-M0099).
Morphological characters on V8 medium: Previously reported in Nishikawa & Nakashima (2013) and Ichinose et al. (2015).
Colony characteristics on PDA after 7 d at 25 °C: Fast-growing, reaching an average of 80 ± 1.8 mm diam; aerial hypha cottony, grayish green to black, with white margins; reverse center dark green to black; sporulation sparse; diffusible pigment absent.
Sexual morph: Not observed.
Natural hosts: Datura (including Brugmansia), Capsicum, Nicandra, Petunia, and Solanum nigrum (Solanaceae) (Rao 1969, Zhang 2003, Simmons 2007, Nishikawa & Nakashima 2013).
Symptoms: Leaf spots appear on Datura, and are vein-limited circular to irregular, straw-yellow to pale brown with a gray center, distinct at their borders, and are scattered, but become enlarged and confluent (Nishikawa & Nakashima 2013).
Experimental host range: Pathogenic to Datura (tribe Datureae, Solanoideae) and Capsicum (tribe Capsiceae, Solanoideae), and occasionally weakly or opportunistic to Petunia and Solanum (Nishikawa & Nakashima 2013).
Distribution: Worldwide, including Asia (China, India, Israel, Japan, Myanmar, Nepal, Pakistan, and Taiwan), Europe (Bulgaria, Croatia, Cyprus, Germany, Italy, Latvia, Macedonia, Poland, Portugal, Romania, Serbia, Spain, and Switzerland), North and Latin America (Cuba, El Salvador, USA, and Venezuela), Africa (Ethiopia, Ghana, Kenya, Mozambique, Nigeria, Rhodesia, South Africa, Sudan, Tanzania, Uganda, Zambia, and Zimbabwe), and the Pacific (Australia and New Zealand) (Sawada 1944, Rao 1969, Ellis 1971, Richardson 1990, Crous et al. 2000, Zhang 2003, Woudenberg et al. 2014, Ichinose et al. 2015, Farr & Rossman 2018).
Distinctive features: Large-spored species with filamentous but clear false beaks, which are usually unbranched, colored, significantly elongated, and often exceed 4 µm in width. Conidial bodies are pale brown, with longitudinal septa in common. Colonies on PDA medium released no pigment, which is unique to the species among related species in sect. Porri. This species is pathogenic to Datura and Capsicum, and is phylogenetically recognizable via its ITS (Fig. 2), gapdh, tef1, rpb2, Alt a 1, and act sequences (data not shown).
Notes: Woudenberg et al. (2014) synonymized A. capsici based on the combined phylogeny of its ITS, gapdh, tef1, rpb2, and Alt a 1 sequences, and then inoculation tests conducted by Nishikawa & Nakashima (2013) also supported this taxonomic classification. In addition, Nishikawa & Nakashima (2013) suggested that A. daturicola was also a probable synonym.
Alternaria cucumerina (Ellis & Everh.) J.A. Elliott, Amer. J. Bot. 4: 472. 1917. Fig. 34.
Fig. 34.

Morphological features of Japanese isolates of Alternaria cucumerina (AC106) on V8 juice agar medium. A–E. Conidia. F, G. Colored beaks. H. Conidiophore. I. Culture on potato-dextrose agar medium (upper = surface, lower = reverse). Scale bars (A–H) = 25 µm.
Basionym: Macrosporium cucumerinum Ellis & Everh., Proc. Acad. Nat. Sci. Philadelphia 47: 440. 1895.
Synonym: Alternaria loofahae E.G. Simmons & Aragaki, CBS Biodiversity Ser. (Utrecht) 6: 316. 2007.
Typus: USA, New Mexico, Las Cruces, on leaves of Cucumis melo, Aug. 1895, E.O. Wooton. (not specified). Lectotype, USA, New Mexico, Las Cruces, on leaves of C. melo, Aug. 1895, E.O. Wooton, PH (designated in Simmons 2007).
Ex-type culture: Unknown.
Additional materials examined: Japan, Niigata Prefecture, Sado, on leaves of Cucurbita maxima, 27 Jul. 2010, Y. Makizumi, living culture AC105; ibid., 30 Jul. 2010, Y. Makizumi, living culture AC106.
Morphological characters on V8 medium: Conidiophores moderately long and broad, 69–109 × 5–7 µm. Conidia usually solitary, but occasionally in chains of two, 56–411 µm in total length. Conidial bodies subcylindrical to broadly obclavate and oblong, 36–106 × 13–28 μm, with 4–15 transverse and 2–12 (often complicated) longitudinal septa, brown to dark brown with a smooth surface. Filamentous beaks almost straight, unbranched, 16–305 × 1–2 µm, pale brown and conspicuously distinguishable, bordering the conidial body.
Colony characteristics on PDA after 7 d at 25 °C: Fast-growing, reaching an average of 87 ± 0.7 mm diam; aerial hypha cottony, white to pale gray; reverse center dark green to black; sporulation sparse; diffusible pigment absent to occasionally bright yellow to pale orange.
Sexual morph: Not observed.
Natural hosts: Cucurbitaceae (Benincasa, Citrullus, Cucumis, Cucurbita, Lagenaria, Luffa, and Sicyos), as well as occasionally reported on Asimina (Annonaceae), Cyamopsis and Phaseolus (Fabaceae) (Ellis 1971, Woudenberg et al. 2014, Farr & Rossman 2018).
Symptoms: Leaf spots appear on Cucurbita, and are dark brown to black with grayish eye at center, subcircular to angular with a distinct border, 1–5 mm diam, becoming confluent.
Distribution: Worldwide, including Asia (China, India, Japan, Korea, Nepal, Pakistan, and Thailand), Europe (Bulgaria, Cyprus, France, Germany, Norway, Romania, Russia, Spain, Switzerland, Turkey, and UK), North and Latin America (Canada, Chile, Cuba, El Salvador, Haiti, Jamaica, Mexico, Peru, Trinidad and Tobago, USA, and Venezuela), Africa (Ghana, Kenya, Libya, Mozambique, Nigeria, Rhodesia, Sierra Leone, South Africa, Sudan, Tanzania, Uganda, Zambia, and Zimbabwe), and the Pacific (Australia and New Zealand) (Benjamin & Slot 1969, Ellis 1971, Yu 2001, Zhang 2003, Gannibal 2011, Woudenberg et al. 2014, Farr & Rossman 2018).
Distinctive features: Large-spored species with filamentous colored beaks, which are usually unbranched and do not exceed 3 µm in width. Conidial bodies are broadly obclavate to oblong, often with complicated longitudinal septa. Colonies on PDA often release yellow to pale orange pigment into the medium. This species is generally characterized by pathogenicity to Cucurbitaceae (Ellis 1971, Yu 2001, Zhang 2003, Simmons 2007), and is phylogenetically recognizable via gapdh, rpb2, tef1 (Fig. 1), and Alt a 1 sequences (data not shown).
Alternaria dauci (J.G. Kühn) J.W. Groves & Skolko, Canad. J. Res., Sect. C, Bot. Sci. 22(5): 222. 1944. Fig. 35.
Fig. 35.

Morphological features of Japanese isolates of Alternaria dauci (MUCC 1684) on V8 juice agar (V8) medium. A–G. Conidia with filamentous beaks. H. Conidiophores. I. Sporulation on surface of V8 medium. J. Culture on potato-dextrose agar medium (upper = surface, lower = reverse). Scale bars (A–H) = 25 µm.
Basionym: Sporidesmium exitiosum var. dauci J.G. Kühn, Hedwigia 1: 91. 1855.
Synonyms: Polydesmus exitiosus var. dauci (J.G. Kühn) J.G. Kühn, Die Krankheiten der Kulturgewächse, ihre Ursachen und ihre Verhütung: 165. 1858.
Macrosporium dauci (J.G. Kühn) Rostr., Tidsskr. Landoekon. ser. 5, 7: 385. 1888.
Macrosporium carotae Ellis & Langl., J. Mycol. 6(1): 36. 1890.
Alternaria carotae (Ellis & Langl.) J.A. Stev. & Wellman, J. Wash. Acad. Sci. 34: 263. 1944.
Alternaria brassicae var. dauci (J.G. Kühn) Lindau, Rabenh. Krypt.- Fl., Edn 2 (Leipzig) 1(9): 260. 1908. non Alternaria brassicae var. dauci (J.G. Kühn) P.C. Bolle, Meded. Phytopathol. Lab. “Willie Commelin Scholten” 7: 42. 1924, later isonym.
Alternaria porri f. sp. dauci (J.G. Kühn) Neerg, Danish species of Alternaria and Stemphylium: 252. 1945.
Alternaria poonensis Ragunath, Mycopathol. Mycol. Appl. 21: 315. 1963.
Typus: Lectotype, B, on leaves of Daucus carota, slide glass specimen of Sporidesmium exitiosum var. dauci, Gross Krausche p. Bunzlau, Jul., Kühn (designated in Simmons 1995a; appeared to be lost according to Woudenberg et al. 2014). Neotype, Italy, from seed of D. carota, Sep. 1937, P. Neergaard, CBS H-21745 (designated in Woudenberg et al. 2014), culture ex-neotype CBS 111.38.
Additional material examined: Japan, Shizuoka Prefecture, Kakegawa, on leaves of D. carota, Nov. 1998, K. Takebayashi, living cultures MUCC 1684 and AC9.
Morphological characters on V8 medium: Conidiophores moderately long and broad, 36–94 × 6–8 µm. Conidia commonly solitary, 152–448 µm in total length. Conidial bodies oblong to broadly obclavate, 52–100 × 13–31 µm, with 5–11 transverse and 0–8 longitudinal septa, sometimes with distosepta-like structures, brown to dark brown, with a smooth surface. Filamentous beaks straight and elongated, hyaline to subhyaline, unbranched or branched once, septated with distoseptum, 100–368 × 1–3 µm.
Colony characteristics on PDA after 7 d at 25 °C: Slow to moderate-growing, reaching an average of 53.6 ± 3.3 mm diam, variable among strains; aerial hypha cottony, grayish green to dark green, with white margins; reverse center dark green to black; sporulation sparse; diffusible pigment red to reddish brown.
Sexual morph: Not observed.
Natural hosts: Daucus, Coriandrum, Apium (Apiaceae), and Cichorium (Asteraceae), as well as some recorded cases infecting non-Apiaceae families under heterogeneous names as forma speciales of A. dauci (Richardson 1990, Simmons 2007, Woudenberg et al. 2014, Farr & Rossman 2018, Poudel & Zhang 2018).
Symptoms: Spots appear on the leaves and petioles of Daucus, and are circular to subcircular with distinct margins, measuring 1–3 mm diam, which become confluent, resulting in severe leaf blight and causing significant economic losses.
Distribution: Worldwide, including Asia (Cambodia, China, India, Israel, Japan, Korea, Malaysia, Nepal, Pakistan, Philippines, Taiwan, and Thailand), Europe (Austria, Bulgaria, Denmark, Germany, Greece, Finland, France, Italy, Netherlands, Poland, Portugal, Russia, Turkey, and UK), North and Latin America (Barbados, Brazil, Canada, Costa Rica, Cuba, El Salvador, Guatemala, Haiti, Honduras, Jamaica, Mexico, Nicaragua, Panama, Peru, Puerto Rico, Trinidad and Tobago, USA, Venezuela, and Virgin Islands), Africa (Algeria, Congo, Ghana, Guinea, Kenya, Malawi, Mauritius, Morocco, Nigeria, South Africa, Tanzania, Zambia, and Zimbabwe), and the Pacific (Australia, Cook Islands, Fiji, New Zealand, Papua New Guinea, Samoa, and Tonga) (Goto 1927, Kranz 1963, Benjamin & Slot 1969, Rao 1969, Richardson 1990, Crous et al. 2000, Yu 2001, Zhang 2003, Soylu et al. 2004, Lopes & Martins 2008, Delgado 2011, Woudenberg et al. 2014, Farr & Rossman 2018, Ozkilinc et al. 2018, Poudel & Zhang 2018).
Distinctive features: Conidial bodies are oblong to obclavate, with hyaline filamentous beaks that often have a single branch. The conidial morphology of the species is indistinguishable from those of A. porri, but differs in length and width (usually not exceeding 3 µm), and more frequently in their longitudinal septa. Its growth rate on PDA is clearly slower, and colonies produce red pigment. This species is pathogenic to Daucus and some other species in Apiaceae (Boedo et al. 2012), and is phylogenetically recognizable via its ITS (Fig. 2), gapdh, rpb2, Alt a1, and act sequences (data not shown).
Alternaria porri (Ellis) Cif., J. Dept. Agric. Porto Rico 14(1): 30. 1930. Figs 36, 37.
Fig. 36.
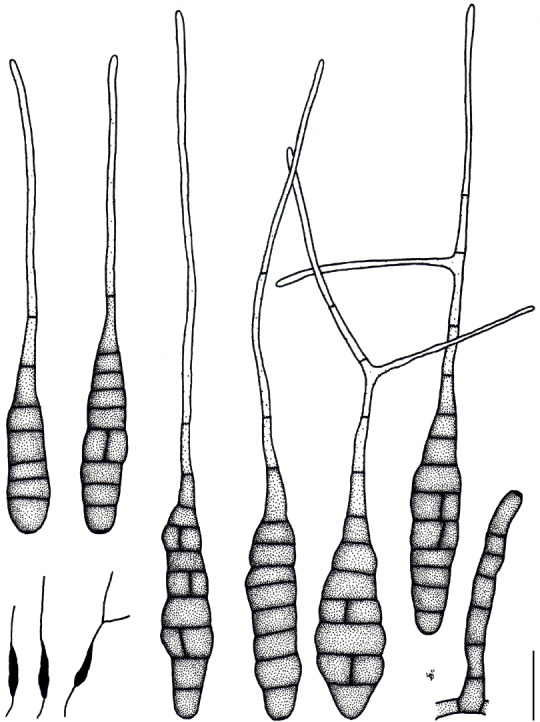
Illustrations of Alternaria porri (AC6). Morphology of conidia and conidiophores, and sporulation patterns (opaque) on V8 juice agar medium. Scale bar = 25 µm.
Fig. 37.

Morphological features of Japanese isolates of Alternaria porri (MUCC 1688) on V8 juice agar medium. A–G. Conidia with filamentous beaks. H. Conidiophores. I. Culture on potato-dextrose agar medium (upper = surface, lower = reverse). J. Natural symptoms on Allium. Scale bars (A–H) = 25 µm.
Basionym: Macrosporium porri Ellis, in Cooke and Ellis, Grevillea 8 (45): 12. 1879.
Synonyms: Alternaria porri (Ellis) Sawada, Rep. Dept. Agric. Gov. Res. Inst. Formosa 61: 92. 1930.
Alternaria porri (Ellis) Neerg., Aarsberet. J. E. Ohlsens Enkes Plantepat. Lab. 3: 5. 1938.
Alternaria allii Nolla, Phytopathology 17: 118. 1927.
Alternaria vanuatuensis E.G. Simmons & C.F. Hill, CBS Biodiversity Ser. (Utrecht) 6: 260. 2007.
Typus: on Allium porrum (holotype not specified). Lectotype, USA, New Jersey, Newfield, on leaves of A. porrum, Sep. 1878, Ellis, NY (designated in Simmons 2007). Epitype, USA, New York, Orange County, from leaf of Allium cepa, 1996, M.J. Yáñes Morales, CBS H-21746 (designated in Woudenberg et al. 2014), culture ex-epitype CBS 116699 = EGS 48.152.
Additional materials examined: Japan, Shizuoka Prefecture, Kakegawa, on leaves of Viola × wittrockiana, 24 Dec. 2003, J. Nishikawa, living culture AC2; ibid., on leaves of Calibrachoa sp., 23 Apr. 2004, J. Nishikawa, MUMH 11670 and MUMH 11699, living culture AC6; ibid., on leaves of Allium fistulosum, 7 Oct. 2004, J. Nishikawa, MUMH 11673, living culture MUCC 1688; Saitama Prefecture, on leaves of A. fistulosum, 1 Nov. 2004, J. Nishikawa, living culture AC15; Gunma Prefecture, Takasaki, on leaves of A. fistulosum, 16 Mar. 2005, J. Nishikawa, living cultures AC16 and AC17; Gunma Prefecture, Tomioka, on leaves of A. fistulosum, 6 Oct. 2006, J. Nishikawa, MUMH 11677, living culture MUCC 1698; Chiba Prefecture, Mobara, on leaves of A. fistulosum, 24 Oct. 2006, J. Nishikawa, living culture AC32; Shizuoka Prefecture, Kakegawa, from seeds of Eustoma exaltatum subsp. russellianum, 20 Mar. 2007, Y. Makizumi, MUMH 11692, living culture MUCC 1702; Tokyo, Setagaya, from seeds of A. fistulosum, 7 Jul. 2001, J. Nishikawa, living culture AC68.
Morphological characters on V8 medium: Conidiophores moderately long and broad, 35–139 × 6–11 µm. Conidia commonly solitary, 75–351 µm in total length. Conidial bodies subcylindrical to oblong, pale brown to brown, with smooth surface, 38–114 × 10–26 µm with 3–12 transverse and 0–5 longitudinal septa, sometimes with distosepta-like structures. Filamentous beaks straight to slightly curved, hyaline, 30–248 × 2–4 µm, unbranched or often branched 1–2 times, septated with distoseptum. Conidial morphology on lesions similar to those on V8 medium, though usually short-beaked.
Colony characteristics after 7 d at 25 °C: Fast-growing, reaching an average of 78.6 ± 1.1 mm diam; aerial hypha cottony, white to grayish green, sometimes with white margins; reverse center dark green; diffusible pigment bright yellow to orange or reddish brown.
Sexual morph: Not observed.
Natural hosts: Allium spp. (Amaryllidaceae) are the most common hosts, although there are reports of the following serving as occasional hosts: Acalypha (Euphorbiaceae), Apium (Apiaceae), Calendula, Gerbera, and Tagetes (Asteraceae), Clarkia (Onagraceae), Dichondra and Ipomoea (Convolvulaceae), Gossypium and Mucuna (Malvaceae), Peganum (Nitrariaceae), Scabiosa (Dipsacaceae), and Solanum (Solanaceae) (Kranz 1963, Rao 1969, Richardson 1990, Crous et al. 2000, Ye et al. 2013, Farr & Rossman 2018).
Symptoms: Leaf spots on Allium are circular to long elliptical, distinct sooty spots, often with purple-stained appearance, measuring 7–50 mm diam. Caespituli were frequently observed on lesions.
Experimental host range: Pathogenic to Allium, but not to leaves of Ageratum, Calibrachoa, Capsicum, Gentiana, Petunia, Solanum, Nicotiana, and Viola (data not shown).
Distribution: Worldwide, including Asia (Brunei, China, India, Indonesia, Iraq, Israel, Japan, Korea, Malaysia, Myanmar, Nepal, Pakistan, Philippines, Taiwan, Thailand, Uzbekistan, and Vietnam), Europe (Austria, Bulgaria, Denmark, France, Germany, Greece, Italy, Netherlands, Poland, Portugal, Romania, Russia, Slovakia, and UK), North and Latin America (Argentina, Brazil, Canada, Colombia, Costa Rica, Cuba, Dominican Republic, El Salvador, Guatemala, Haiti, Honduras, Jamaica, Mexico, Nicaragua, Panama, Puerto Rico, USA, Venezuela, and Virgin Islands), Africa (Egypt, Ethiopia, Ghana, Guinea, Kenya, Libya, Malawi, Mauritius, Nigeria, Rhodesia, South Africa, Tanzania, Uganda, Zambia, and Zimbabwe), and the Pacific (Australia, Fiji, New Caledonia, New Zealand, Papua New Guinea, Tonga, and Vanuatu) (Yoshii 1929a, Rao 1969, Ellis 1971, Stevenson 1975, Richardson 1990, Aveling & Naude 1992, Koike & Henderson 1998, Crous et al. 2000, Yu 2001, Zhang 2003, Hall et al. 2007, Simmons 2007, Delgado 2011, Woudenberg et al. 2013, 2014, Farr & Rossman 2018).
Distinctive features: Large-spored species with hyaline filamentous beaks, which are often branched and exceeded 3 µm in width. Conidial bodies are subcylindrical, with relatively fewer longitudinal septa. Colonies grown on PDA medium released bright yellow to reddish brown pigment. This species is pathogenic to Allium, and is phylogenetically recognizable via its ITS (Fig. 2), gapdh, tef1, rpb2, Alt a 1, and act sequences (data not shown).
Notes: Woudenberg et al. (2014) recognized A. allii, which is a morphospecies with multiple branched beaks (Simmons 2007), as a distinct taxon based on the combined phylogeny of ITS, gapdh, rpb2, tef1, and Alt a 1 sequences. However, the morphology of isolates that clustered in the A. allii clade was not always distinguishable in beak branching (Figs 36, 37), and results of phylogenetic analysis based on ITS, gapdh, tef1, Alt a 1, and act sequences individually did not clearly support this species as a unique Allium pathogen (data not shown). Therefore, we regarded A. allii as a synonym of A. porri here. This species is also ubiquitous, and frequently found on non-host plants.
Alternaria zinniae M.B. Ellis, Mycol. Pap. 131: 22. 1972. Fig. 38.
Fig. 38.

Morphological features of Japanese isolates of Alternaria zinniae (MUCC 1704) on potato-carrot agar medium. A–E. Conidia. F. Conidiophores. G. Culture on potato-dextrose agar medium (upper = surface, lower = reverse). H. Natural symptoms on seedlings of Zinnia. Scale bars (A–F) = 25 µm.
Synonym: Alternaria zinniae H. Pape, Angew. Bot. 24: 61. 1942, nom. inval. (no Latin; ICN Art. 39.1).
Typus: holotype specimen not specified. Lectotype, USA, New York, Ithaca, on Zinnia elegans, 28 Sep. 1942, A.W. Dimock, IMI 1037 (designated in Simmons 2007; as holotype in Simmons 1997).
Ex-type culture: Unknown.
Additional materials examined: Japan, Nagano Prefecture, Tomi, on leaves of Zinnia hybr., 6 Jul. 2007, J. Nishikawa, MUMH 11680, living culture MUCC 1704; Nagano Prefecture, Azumino, on leaves of Zinnia hybr., Aug. 2010, Y. Makizumi, living culture AC107; Shizuoka Prefecture, Kakegawa, on Z. elegans, 16 Mar. 2011, Y. Makizumi, living culture AC108; Nagano Prefecture, Azumino, on Z. elegans, 31 May 2011, Y. Makizumi, living culture AC109.
Morphological characters on V8 medium: Conidiophores moderately long and broad, 60–183 × 6–8 µm. Conidia usually solitary, but occasionally in chains of two, 109–318 µm in total length. Conidial bodies subcylindrical to oblong, 74–119 × 20–33 μm, with 9–16 transverse and 6–14 (often complicated) longitudinal septa, olive brown to brown, with smooth to minutely verrucose surface. Filamentous beaks almost straight, unbranched, pale brown, conspicuously distinguishable and border the conidial body, 33–213 × 1–3 µm.
Colony characteristics on PDA after 7 d at 25 °C: Moderate-growing, reaching an average of 60.4 ± 5.6 mm diam, variable among strains; aerial hypha cottony, white to pale gray, with white margins; reverse center dark green to black; diffusible pigment yellow to pale orange.
Sexual morph: Not observed.
Natural hosts: Usually Zinnia and the other Asteraceae plants (Ageratum, Bidens, Blumea, Calendula, Callistephus, Carthamus, Coreopsis, Cosmos, Dahlia, Echinops, Eclipta, Eupatorium, Gaillardia, Galinsoga, Gerbera, Glebionis, Helianthus, Kleinia, Parthenium, Rudbeckia, Sphaeranthus, Spilanthes, Tagetes, Tithonia, Volutaria, and Xanthium) (Neergaard 1945, Rao 1969, Ellis 1976, Richardson 1990, Farr & Rossman 2018). Records suggest that it may also infect Impatiens (Balsaminaceae), Nicotiana (Solanaceae), and Papaver (Papaveraceae) (Neergaard 1945, Richardson 1990).
Symptoms: Leaf spots on seedlings of Zinnia are brown, circular to irregular, measuring 5–10 mm diam, becoming enlarged and confluent.
Distribution: Worldwide, including Asia (China, Brunei, India, Indonesia, Japan, Korea, Malaysia, Myanmar, Nepal, and Pakistan), Europe (Armenia, Austria, Cyprus, Denmark, France, Germany, Hungary, Italy, Latvia, Netherlands, Norway, Poland, Portugal, Romania, and UK), North and Latin America (Brazil, Canada, Guyana, Jamaica, and USA), Africa (Egypt, Ethiopia, Ghana, Guinea, Kenya, Libya, Malawi, Mauritius, Rhodesia, Sierra Leone, South Africa, Sudan, Tanzania, Uganda, Zambia, anThis section is morphologically characterized by larged Zimbabwe), and the Pacific (Australia, New Caledonia, New Zealand, and Tonga) (Kranz 1963, Rao 1969, Ellis 1976, Richardson 1990, Simmons 1997, Crous et al. 2000, Yu 2001, Zhang 2003, Woudenberg et al. 2014, Farr & Rossman 2018).
Distinctive features: Large-spored species with filamentous colored beaks as with those of A. cucumerina. Conidial bodies are oblong, often with complicated longitudinal septa. Colonies grown on PDA medium sometimes release yellow to pale orange pigment into the medium. This species is generally characterized by selective pathogenicity to Zinnia and some other Asteraceae plants (Neergaard 1945, Zhang 2003), and is phylogenetically recognizable via gapdh, rpb2, tef1 (Fig. 1), Alta 1, and act sequences (data not shown).
Note: Since there are a wide range of records listing Asteraceae as a host, further pathological studies within Asteraceae, besides Zinnia, are needed to characterize this species.
Section Pseudoulocladium Woudenb. & Crous, Stud. Mycol. 75: 201. 2013.
The four species assigned to this section are characterized by simple or branched, short, and geniculate conidiophores, and catenation of mostly 3-septate conidia (Runa et al. 2009, Woudenberg et al. 2013). Gannibal & Lawrence (2018b) listed additional species in this section, A. lanuginosa and A. sylvestris, afterward.
Alternaria chartarum Preuss, Bot. Zeitung 6: 412. 1848. Fig. 39.
Fig. 39.
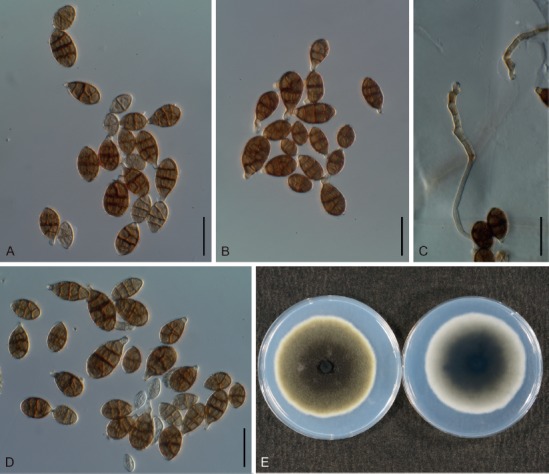
Morphological features of Japanese isolates of Alternaria chartarum (MAFF 246888). A–D. Conidia and conidiophores on potato-carrot agar medium. E. Culture on potato-dextrose agar medium (left = surface, right = reverse). Scale bars (A–D) = 25 µm.
Basionym: Sporidesmium polymorphum var. chartarum (Preuss) Cooke, Fungi Brit. Exs., ser. 2: 329. 1875.
Synonyms: Ulocladium chartarum (Preuss) E.G. Simmons, Mycologia 59: 88. 1967.
Alternaria stemphylioides Bliss, Mycologia 36: 538. 1944.
Alternaria chartarum f. stemphylioides (Bliss) P. Joly, Encycl. Mycol. (Paris) 33: 161. 1964.
Typus: Germany, Hoyerswerda, holotype in B, on paper, Preuss, Klotzsch’s Herb. vivum mycol. no. 1284. Epitype, Canada, Saskatchewan, from Populus plywood, Jul. 1957, S.J. Hughes, CBS H-19059 (designated in de Hoog & Horré 2002), culture exepitype CBS 200.67 = ATCC 18044 = DAOM 59616b = IMI 124943 = MUCL 18564 = QM 8328.
Additional material examined: Japan, Tokyo, Setagaya, from seeds of Capsicum annuum, 8 Dec. 2000, J. Nishikawa, living culture MAFF 246888.
Morphological characters on PCA medium: Conidiophores solitary and relatively short, 18–95 × 3–5 μm, pale brown to brown, with polytretic pores at the apex, 2–4 geniculate bends, frequently proliferating at the upper nodes. Conidia in short chains of 3–8, frequently with lateral branches, brown to dark brown, ellipsoid to obclavate, smooth to roughened, 13–29 × 8–15 μm, with 1–4 (mostly 3) transverse and 0–4 longitudinal septa. Secondary conidiophores (false beaks) at the apical end and median of conidium, short, mostly single-celled, sometimes proliferate and branched.
Colony characteristics on PDA after 7 d at 25 °C: Moderate-growing, reaching 60.8 ± 3.1 mm diam; aerial hypha cottony to sparse, dark green to greenish brown, with white margins; reverse center dark green to pale gray; sporulation abundant; diffusible pigment absent.
Sexual morph: Not observed.
Natural hosts: Saprophytic, but there are records of pathogenicity to Quercus, Vaccinium, and Lippia (Vannini & Vettraino 2000, Starast et al. 2009, Zarandi & Sharzei 2015).
Distribution: Worldwide, including Asia (China, India, Iran, Iraq, Israel, Japan, Kuwait, Pakistan, and Saudi Arabia), Europe (Lithuania, Poland, Russia, and UK), North and Latin America (Canada, Nicaragua, Uruguay, and USA), South Africa, and Australia (Rao 1969, Ellis 1976, Phillips et al. 1979, Rossman & Lu 1980, Watanabe et al. 1986, Bettucci et al. 1997, Chen et al. 2002, Nishikawa et al. 2006, Delgado 2011, Kowalski & Andruch 2012, Woudenberg et al. 2013, Zarandi & Sharzei 2015, Barkat et al. 2016, Farr & Rossman 2018).
Distinctive features: Short conidiophores are geniculate and proliferate frequently; obclavate conidia commonly appear in chains, with three transverse septa. This species may be phylogenetically recognizable via ITS (Fig. 2), gapdh, tef1, Alt a 1, and act sequences (data not shown).
Notes: The results of the gapdh and tef1 phylogeny also suggested that isolates of A. aspera, A. chartarum, A. concatenata, and A. septospora must be conspecific (data not shown), and morphological differences between these species were unclear based on their original descriptions (Simmons 1967, 2004, Xue & Zhang 2007). This species has often been misidentified as A. alternata owing to its conidial catenation (Simmons 1967, de Hoog & Horré 2002, Runa et al. 2009).
Section Radicina D.P. Lawr. et al., Mycologia 105: 541. 2013.
Five species were recognized in this section (Lawrence et al. 2013, Woudenberg et al. 2013), which is morphologically characterized by medium-sized, beakless conidia. All recognized species in this section were sourced from Apiaceae. Two species, A. petroselini and A. radicina, are distributed in Japan (Yoshii 1929b, Nishikawa & Nakashima 2013), and the former was examined in the present study.
Alternaria petroselini (Neerg.) E.G. Simmons, in Ellis, More dematiaceous Hyphomycetes (Kew): 417. 1976. Fig. 40.
Fig. 40.

Morphological features of Japanese isolates of Alternaria petroselini (MAFF 243057). A–C. Conidia on potato-carrot agar (PCA) medium. D, E. Conidiophores on PCA medium. F. Natural symptoms on Petroselinum. Scale bars (A–E) = 25 µm.
Basionym: Stemphylium petroselini Neerg., Zentralbl. Bakteriol., 2. Abt. 104: 411. 1942.
Synonyms: Stemphylium radicinum var. petroselini (Neerg.) Neerg., Danish species of Alternaria and Stemphylium: 357. 1945.
Alternaria radicina var. petroselini (Neerg.) Neerg., Encycl. Mycol. 33: 123. 1964.
Macrosporium cheiranthi f. petroselini Sacc., Rev. Mycol. (Toulouse) 19: 54. 1897.
Alternaria selini E.G. Simmons, Mycotaxon 55: 109. 1995.
Typus: Denmark, from seeds of Petroselinum crispum, 4 Apr. 1941, P. Neergaard, holotype EGS 11.062 in CP, culture presumably ex-holotype CBS 112.41 = EGS 06.196.
Additional material examined: Japan, Shizuoka Prefecture, Kakegawa, on leaves of P. crispum, 27 Apr. 2007, J. Nishikawa, MUMH 11679, living culture MAFF 243057.
Morphological characters on PCA medium: Previously reported in Nishikawa & Nakashima (2013).
Colony characteristics on PDA after 7 d at 25 °C: Fast-growing, reaching 82.1 ± 1.8 mm diam; characteristics previously reported in Nishikawa & Nakashima 2013.
Sexual morph: Not observed.
Natural hosts: Typically, Petroselinum, Coriandrum, and Foeniculum (Apiaceae), but may also occasionally infect Carya (Juglandaceae) (Liu et al. 2013).
Symptoms: Leaf spots on Petroselinum indistinct, sooty brown, water-soaked, and expand to leaf blight (Nishikawa & Nakashima 2013).
Experimental host range: Widely pathogenic within Apiaceae plants, including Ammi, Anethum, Angelica, Anthriscus, Apium, Bupleurum, Coriandrum, Cuminum, Foeniculum, and Petroselinum (Nishikawa & Nakashima 2013).
Distribution: Australia, China, Italy, Japan, Netherlands, Saudi Arabia, Spain, UK, and USA (Ellis 1976, Farrar et al. 2004, Cunnington et al. 2007, Pryor & Asma 2007, Park et al. 2008, Infantino et al. 2009, Bassimba et al. 2012, Liu et al. 2013, Nishikawa & Nakashima 2013, Farr & Rossman 2018).
Distinctive features: Conidia are solitary or appear in short chains and are mostly beakless, and broad-ovoid to long-ellipsoid (but variable in shape and size). This species was widely pathogenic to Apiaceae, but not to Daucus, and is phylogenetically recognizable via its ITS (Fig. 2), rpb2, Alt a 1, and act sequences (data not shown).
Notes: All the sequences of the examined Japanese isolate MAFF 243057 were identical with those of the ex-type isolates, A. petroselini (CBS 112.41) and A. selini (CBS 109382). Based on both phylogenetic analysis and morphological observations, A. selini was never distinguishable from A. petroselini (Fig. 1), and, thus, they are synonymized in the present study.
Section Sonchi D.P. Lawr. et al., Mycologia 105: 542. 2013.
This section is morphologically characterized by large conidia, which are solitary or may appear in short chains with a blunt-tapered false beak. There are only two species assigned to this section (Woudenberg et al. 2013), although Lawrence et al. (2013) included A. brassicae within this section.
Alternaria cinerariae Hori & Enjoji, J. Pl. Protect. (Tokyo) 18(8): 432. 1931. Fig. 41.
Fig. 41.

Morphological features of Japanese isolates of Alternaria cinerariae (MAFF 243059) on V8 juice agar medium. A–H. Conidia. I. Conidiophores. J. Dried culture specimen ex MAFF 243059 (epitype: TNS-F-85448). K. Natural symptoms on Pericallis. Scale bars (A–I) = 25 µm.
Synonym: Alternaria senecionis Neerg., Danish species of Alternaria and Stemphylium: 201. 1945.
Typus: Japan, Chiba Prefecture, Chiba, Chiba Prefect. Agric. Exp. Station, on leaves of Pericallis cruenta, 30 Mar. 1931, and in Apr. to May 1931, S. Enjoji (holotype not specified; not preserved). Lectotype, Japan, Chiba Prefect. Agric. Exp. Station, on Senecio cineraria, 28 Apr. 1931, S. Enjoji, in FU (designated in Simmons 1997). Epitype designated here, Japan, Chiba Prefecture, Narita, on leaves of P. cruenta, 25 Oct. 2002, J. Nishikawa, TNS-F-85448 (dried culture specimen ex MAFF 243059) [MBT 385024], isoepitype MUMH 11691, culture ex-epitype MAFF 243059 = MUCC 1701.
Additional materials examined: Japan, Ibaraki Prefecture, Tsukuba, Kannondai, on leaves of Farfugium japonicum, Nov. 2008, Y. Otani, MUMH 11694, living culture MAFF 241266 = MUCC 1613; ibid., on leaves of Gynura bicolor, Nov. 2008, Y. Otani, MUMH 11695, living culture MAFF 241267 = MUCC 1614; Kanagawa Prefecture, Atsugi, on leaves of Jacobaea maritima, 23 Aug. 2017, Y. Makizumi, living culture MUCC 2504.
Morphological characters on V8 medium: Conidiophores broad, 25–196 × 6–11 µm, often branched but sometimes unbranched.
Conidia solitary to in chains of 2–5(–9), rarely with lateral branches, faintly yellowish-tan to pale brown, smooth, long ellipsoid to obclavate, with a blunt tapered false beak, mostly straight and laterally symmetrical, 18–319 µm in total length, constricted at each transverse septum. Conidial bodies sometimes excessively swollen, 18–295 × 8–63 µm, with 1–14 transverse septa and up to 10 longitudinal septa; false beaks unbranched, up to 80–159 × 5–9 µm, concolorous with body, inconspicuous border with the conidial body. Conidia of ex-epitype culture MAFF 243059 on V8 medium solitary to in chains of 2–3 conidia; conidial bodies 30–138 × 9–46 μm, with 2–12 transverse and up to 10 longitudinal septa; secondary conidiophores up to 123 × 5–9 μm.
Colony characteristics on PDA after 7 d at 25 °C: Moderate-growing, reaching an average of 62.9 ± 3.6 mm diam, variable among strains; aerial hypha cottony, grayish green to dark green, with white margins; reverse center black to dark green; sporulation sparse; diffusible pigment absent.
Sexual morph: Not observed.
Natural hosts: Farfugium, Gynura, Jacobaea, Ligularia, Pericallis, and Senecio (Asteraceae) (Nishikawa & Nakashima 2015).
Symptoms: Leaf spots on Pericallis black, circular to irregular, and 3–10 mm diam, often with a necrotic eye at the center. They appear water-soaked, enlarge, and become confluent.
Experimental host range: Selectively pathogenic to tribe Senecioneae, and experiments suggest weak pathogenicity to Cosmos bipinnatus and Centaurea (Nishikawa & Nakashima 2015).
Distribution: Worldwide, but few records exist; Denmark, Germany, Japan, Korea, New Zealand, South Africa, UK, and USA (Enjoji 1931, Neergaard 1945, Ellis 1976, Richardson 1990, Yu 2001, Simmons 2007, Woudenberg et al. 2013, Nishikawa & Nakashima 2015, Farr & Rossman 2018).
Distinctive features: Conidia are large, solitary or in short chains with a blunt-tapered false beak. Conidiophores are long, broad, and sometimes branching. This species is selectively pathogenic to tribe Senecioneae, which includes genera Senecio, Farfugium and Gynura, and is phylogenetically recognizable via its ITS (Fig. 2), gapdh, rpb2, Alt a 1, and act sequences (data not shown).
Notes: Morphological variations are present between strains, including the appearance of excessively swollen bodies and chlamydospore (microsclerotia) formation (Nishikawa & Nakashima 2015). There is no ex-type culture and few reference isolates, and the epitype originated near the original type locality; therefore, an ex-epitype isolate was designated and deposited for future studies.
Section Ulocladioides Woudenb. & Crous, Stud. Mycol. 75: 204. 2013.
There are ten species and a representative strain of A. botrytis assigned to this section (Woudenberg et al. 2013), which is typified by A. cucurbitae, and consists of a majority of the former Ulocladium spp. Recently, Gannibal & Lawrence (2018b) additionally listed ten species citing Geng et al. (2014). Conidial morphology resembles those of species in sect. Ulocladium (Woudenberg et al. 2013).
Alternaria atra (Preuss) Woudenb. & Crous, Stud. Mycol. 75: 204. 2013. Fig. 42.
Fig. 42.

Morphological features of Japanese isolates of Alternaria atra (MAFF 246889). A–D. Conidia on potato-carrot agar medium. E, F. Conidiophores. G. Culture on potato-dextrose agar medium (left = surface, right = reverse). Scale bars (A–F) = 25 µm.
Basionym: Ulocladium atrum Preuss, Linnaea 25: 75. 1852.
Synonyms: Stemphylium atrum (Preuss) Sacc., Syll. Fung. 4: 520. 1886.
Alternaria abietis Tengwall, Meded. Phytopath. Lab. ‘WCS’ 6: 50. 1924.
Typus: Germany, Hoyerswerda, on Betula pubescens (as Betula alba), Preuss, in B. Epitype, USA, California, from soil, Nov. 1962, P.M.D. Martin, BPI 444871 (designated in de Hoog & Horré 2002), culture ex-epitype CBS 195.67 = ATCC 18040 = IMI 124944 = QM 8408.
Additional materials examined: Japan, Tokyo, Setagaya, from seeds of Raphanus sativus, Jul. 2000, J. Nishikawa, living culture AC86; ibid., from seeds of Brassica oleracea var. capitata, 4 Feb. 2001, J. Nishikawa, living culture AC87; ibid., from seeds of Brassica rapa subsp. pekinensis, 18 Mar. 2001, J. Nishikawa, living culture AC88; ibid., from seeds of A. fistulosum, 7 Jul. 2001, J. Nishikawa, living culture MAFF 246889.
Morphological characters on PCA medium: Conidiophores solitary, usually unbranched and geniculate, frequently proliferating sympodially, pale brown to brown, 23–73 × 3–5 μm, with pores for polytretic sporulation. Conidia commonly solitary, varied from subsphaeroid, obovoid, obclavate to ellipsoid, brown to dark brown, roughened to conspicuously verrucose, 10–33 × 6–17 μm, with (0–)1(–3) transverse and 0–2 longitudinal septa, usually beakless, but sometimes with a secondary conidiophore at the apex. Secondary conidiophores geniculate, 3–38 × 3–5 μm.
Colony characteristics on PDA after 7 d at 25 °C: Moderate-growing, reaching an average of 65.3 ± 3.1 mm diam, variable among strains; aerial hyphae cottony to sparse, grayish green to dark green or black, with white margins; reverse center pale gray to dark green or black; sporulation commonly abundant; diffusible pigment absent.
Sexual morph: Not observed.
Natural hosts: Saprophytic, but a few records suggest pathogenicity to Helianthus and Solanum (Shtienberg 1994, Esfahani 2018).
Distribution: Worldwide, including Asia (China, India, Iran, Israel, Japan, Korea, Kuwait, Pakistan, and Saudi Arabia), Europe (Cyprus, Denmark, Germany, Italy, Netherlands, Poland, Spain, and UK), North and Latin America (Argentina, Canada, Mexico, and USA), Africa (Egypt, Libya, and Sierra Leone), and the Pacific (Australia and New Zealand) (Ellis 1976, Abdel-Hafez 1984, Shtienberg 1994, Heredia et al. 1995, Chen et al. 2002, Lunghini et al. 2013, Esfahani 2018, Farr & Rossman 2018).
Distinctive features: Short conidiophores are geniculate and frequently proliferate; conidia are commonly solitary, but often appear in chains with secondary conidiophores, generally with one transverse septum in a body. This species may be phylogenetically recognizable via its ITS (Fig. 2), gapdh, tef1, rpb2, and Alt a1 sequences (data not shown).
Notes: The multi-locus phylogeny shown in this study (Fig. 1) as well as previous studies (Runa et al. 2009, Woudenberg et al. 2013, Geng et al. 2014) suggested that this section consists of three or more strongly supported subclades, with overlapping conidial morphology. These species were commonly regarded as saprophytes (Rena et al. 2009), and consequently could not be examined under the concept of integrated species recognition in this study. Therefore, we provisionally identified Japanese isolates as A. atra based on their conidial morphology and ITS phylogeny, which may determine species boundaries in other former Ulocladium spp. in sect. Pseudoulocladium and Ulocladium (Fig. 2).
Section Ulocladium (Preuss) Woudenb. & Crous, Stud. Mycol. 75: 206. 2013.
Basionym: Ulocladium Preuss, Linnaea 24: 111. 1851.
Four species typified by A. botrytis were assigned to this section by Woudenberg et al. (2013), and former Sinomyces spp., which is S. alternariae, S. fusoideus and S. obovoideus, may also be included in this section. Gannibal & Lawrence (2018b) additionally listed A. manihoticola in this section. Two species, A. botrytis and A. oudemansii, are found in Japan (Katumoto 2010), though there are a few morphological differences between the species in this section (Simmons 1967, Runa et al. 2009).
Alternaria botrytis (Preuss) Woudenb. & Crous, Stud. Mycol. 75: 206. 2013. Fig. 43.
Fig. 43.

Morphological features of Japanese isolates of Alternaria botrytis (MAFF 246887). A–E. Conidia on potato-carrot agar medium. F, G. Conidiophores producing conidia. H. Culture on potato-dextrose agar medium (upper = surface, lower = reverse). Scale bars (A–G) = 25 µm.
Basionym: Ulocladium botrytis Preuss, Linnaea 24: 111. 1851.
Synonyms: Stemphylium botryosum Wallr. var. ulocladium Sacc., Syll. Fung. 4: 522. 1886.
Stemphylium botryosum Wallr. var. botrytis (Preuss) Lindau, Rabenh. Krypt.-Fl., Edn 2 1(9): 219. 1908.
Alternaria oudemansii (E.G. Simmons) Woudenb. & Crous, Stud. Mycol. 75: 206. 2013.
Ulocladium oudemansii E.G. Simmons, Mycologia 59: 86. 1967.
Typus: Germany, Hoyerswerda, on wood sliver of Quercus, holotype in B. Epitype, USA, Cambridge, Massachusetts, contaminant (air), CBS H-19057 (designated in de Hoog & Horré 2002), culture ex-epitype CBS 197.67 = ATCC 18042 = IMI 124942 = MUCL 18556 = QM 7878.
Additional material examined: Japan, Shizuoka Prefecture, Kakegawa, from rhizomes of Asparagus officinalis, 8 Apr. 2008, J. Nishikawa, living culture MAFF 246887.
Morphological characters on PCA medium: Conidiophores solitary, often branched, geniculate, frequently proliferate sympodially, pale brown to brown, 48–145 × 2–4 μm, with pores for polytretic sporulation. Conidia commonly solitary, brown to dark brown, roughened to conspicuously verrucose, obovoid to ellipsoid, beakless, 13–30 × 8–17 μm, with (1–)3 transverse and 0–3 longitudinal septa.
Colony characteristics on PDA after 7 d at 25 °C: Moderate-growing, reaching 68.1 ± 0.9 mm diam; aerial hypha commonly sparse, green to greenish brown, with white margins; reverse center black to dark green; sporulation abundant; diffusible pigment absent.
Sexual morph: Not observed.
Natural hosts: Saprophytic (recorded on Pinus, Alnus, Betula, etc., but no records exist suggesting pathogenicity) (Wicker & Yokota 1982, Farr & Rossman 2018).
Distribution: China, Egypt, Germany, India, Japan, Kuwait, Pakistan, Poland, Russia, Thailand, UK, Uruguay, and USA (Ellis 1971, Tokumasu et al. 1994, Alonso et al. 2011, Farr & Rossman 2018).
Distinctive features: Long conidiophores are geniculate and proliferate. Conidia are solitary and typically obovoid, usually with three transverse septa. This species is phylogenetically recognizable via its ITS (Fig. 2), gapdh, tef1, rpb2, Alt a 1, and act sequences (data not shown).
Notes: Phylogenetic analysis conducted during the present study, as well as morphological similarity, suggest that this species is conspecific with A. alternariae and A. oudemansii (Runa et al. 2009, Woudenberg et al. 2013) (Fig. 1). However, the examined isolate of A. alternariae (CBS 126989 = EGS 46.004) is not an authentic isolate of the species (Wang et al. 2011), and therefore, just A. oudemansii is synonymized herein. This species has already been observed (as A. botrytis or A. oudemansii) on pine and Japanese cedar seeds (Wicker & Yokota 1982, Watanabe et al. 1986, Watanabe & Sato 1988).
Monotypic lineages
Woudenberg et al. (2013) recognized six species as single species not assigned to hitherto known sections, namely A. argyranthemi, A. brassicae, A. dennisii, A. helianthiinficiens, A. soliaridae, and A. thalictrigena. Lawrence et al. (2016) recognized an additional two monotypic lineages, A. peucedani and A. thlaspis. Among these, A. brassicae is already known in Japan, and a novel species isolated from Bupleurum is also newly described here as a ninth monotypic lineage.
Alternaria brassicae (Berk.) Sacc., Michelia 2: 172. 1880. Figs 44, 45.
Fig. 44.
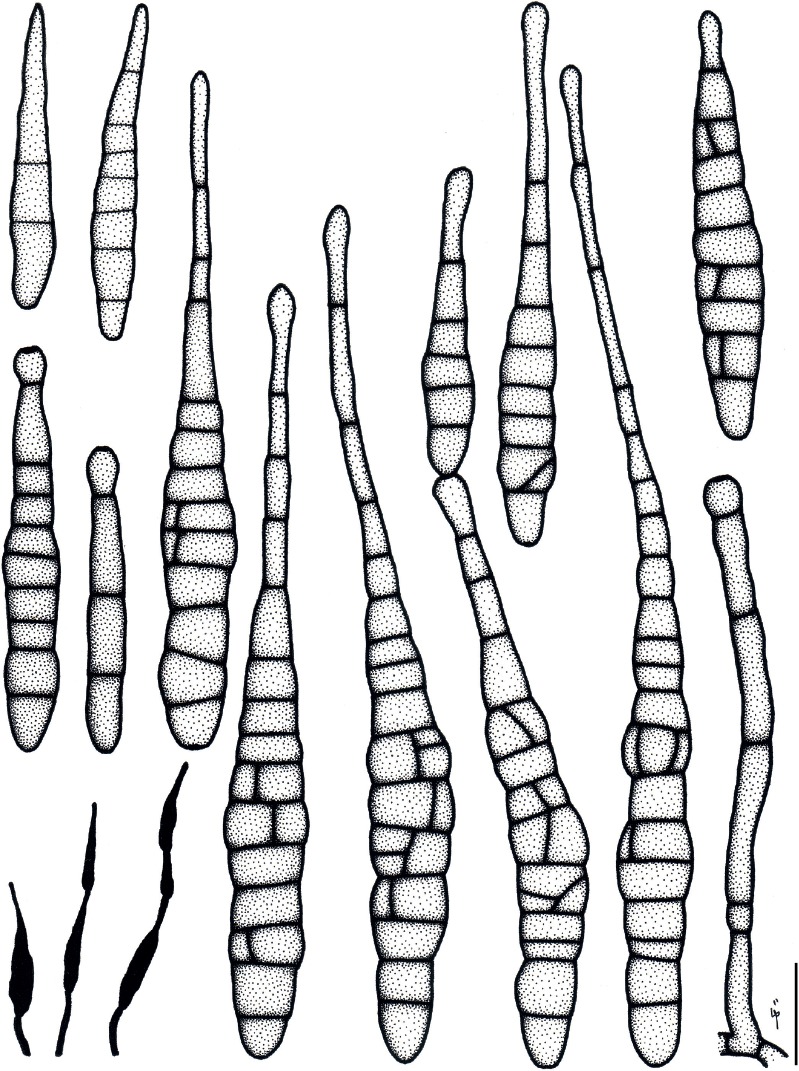
Illustrations of Alternaria brassicae (MAFF 240791). Morphology of conidia and conidiophores, and sporulation patterns (opaque) on V8 juice agar medium. Scale bar = 25 µm.
Fig. 45.

Morphological features of Japanese isolates of Alternaria brassicae (MAFF 240791). A–H. Conidia and conidiophores on V8 juice agar medium. I. Culture on potato-dextrose agar medium (upper = surface, lower = reverse). Scale bars (A–H) = 25 µm.
Basionym: Macrosporium brassicae Berk., in Smith, Engl. Fl., Fungi 5(2): 339. 1836.
Additional synonyms in Simmons (2007).
Typus: UK, Northamptonshire, Kings Cliffe, on decaying leaves of Brassica oleracea var. capitata, M.J. Berkeley (holotype specimen unknown according to Simmons 1995a). Neotype, UK, Essex, on leaves of B. oleracea var. capitata, 16 Oct. 1966, E.G. Simmons, IMI 369156 (designated in Simmons 1995a).
Ex-type culture: Unknown.
Additional materials examined: Japan, Shizuoka Prefecture, Kakegawa, from seeds of Brassica rapa, 15 Aug. 2006, J. Nishikawa, living culture AC29; Ibaraki Prefecture, Tsukuba, on leaves of Raphanus sativus, Jul. 2007, T. Sato, living culture MAFF 240791; Chiba Prefecture, Narita, Minami-misatozuka, on leaves of R. sativus, 13 Nov. 2009, J. Nishikawa, MUMH 11684, living culture MUCC 1615.
Morphological characters on V8 medium: Conidiophores pale brown to brown, broad, 38–183 × 6–11 µm. Conidia solitary to in short chains of 1–2, pale brown to brown, subcylindrical to oblong, with blunt-tapered beaks, 40–237 µm in total length. Conidial bodies 33–160 × 8–33 µm, with 1–10 transverse and 0–9 longitudinal septa, commonly smooth. Beaks straight, not filamentous, unbranched, concolorous with the bodies, 6–121 × 3–10 µm.
Colony characteristics on PDA after 7 d at 25 °C: Slow-growing, reaching an average of 37.6 ± 1.6 mm diam; aerial hypha cottony, white to pale gray; reverse center black to dark green; sporulation sparse; diffusible pigment absent.
Sexual morph: Not observed.
Natural hosts: Brassicaceae (Arabis, Armoracia, Brassica, Bunias, Camelina, Cochlearia, Crambe, Descurainia, Eruca, Eutrema, Iberis, Lepidium, Lunaria, Neslia, Radicula, Raphanus, Rorippa, Sinapis, Sisymbrium, and Sisymbrium), Cucumis sativa and Cucurbita pepo (Cucurbitaceae), and Beta (Amaranthaceae) are correct source plants according to Simmons (2007). All other recorded hosts reported by Farr & Rossman (2018) may be listed under the names of each formae and variety of A. brassicae.
Symptoms: Small, black spots appear on the leaves and petioles of Raphanus. They are 5 mm diam, circular to zonate, with a necrotic eye at the center, becoming enlarged and confluent.
Experimental host range: Strongly pathogenic to Brassicaceae, including Diplotaxis, Iberis, and Nasturtium; weakly pathogenic to Eutrema, Lobularia, and Matthiola; almost non-pathogenic to Aubrieta, Capsella, and non-Brassicaceae plants (Table 5).
Distribution: Worldwide, including Asia (Bangladesh, Cambodia, China, India, Indonesia, Iraq, Japan, Korea, Kyrgyzstan, Malaysia, Myanmar, Nepal, Oman, Pakistan, Philippines, Singapore, Taiwan, Thailand, and Turkmenistan), Europe (Armenia, Austria, Azerbaijan, Bulgaria, Cyprus, Czech, Denmark, France, Germany, Greece, Italy, Latvia, Macedonia, Netherlands, Poland, Romania, Russia, Serbia, Slovenia, Spain, Turkey, and UK), North and Latin America (Argentina, Bolivia, Brazil, Canada, Chile, Colombia, Costa Rica, Cuba, Dominican Republic, El Salvador, Guatemala, Haiti, Honduras, Jamaica, Mexico, Nicaragua, Panama, Peru, Puerto Rico, Trinidad and Tobago, Uruguay, Venezuela, Virgin Islands, and USA), Africa (Egypt, Ethiopia, Kenya, Malawi, Mauritius, Morocco, Nigeria, South Africa, Sudan, Tanzania, Zambia, and Zimbabwe), and the Pacific (Australia, New Zealand, and Papua New Guinea) (Farr & Stevenson 1963, Benjamin & Slot 1969, Rao 1969, Richardson 1990, Jasalavich et al. 1995, Koike 1996, Koike & Molinar 1997, Crous et al. 2000, Cho et al. 2001, Yu 2001, Zhang 2003, Gaetan & Madia 2005, You et al. 2005, Simmons 2007, Caesar & Lartey 2009, Gannibal & Gasich 2009, Woudenberg et al. 2013, Blagojević et al. 2015, van de Wouw et al. 2016, Farr & Rossman 2018).
Distinctive features: Large spores with blunt-tapered false beaks, and slow-growing on PDA medium. This species is widely pathogenic to Brassicaceae, including Eutrema, but not to Aubrieta and Capsella. It is phylogenetically recognizable via its ITS (Fig. 2), gapdh, tef1, rpb2, Alt a1, and act sequences (data not shown).
Alternaria triangularis Jun. Nishikawa & C. Nakash., sp. nov. MycoBank MB829137. Figs 46, 47.
Fig. 46.
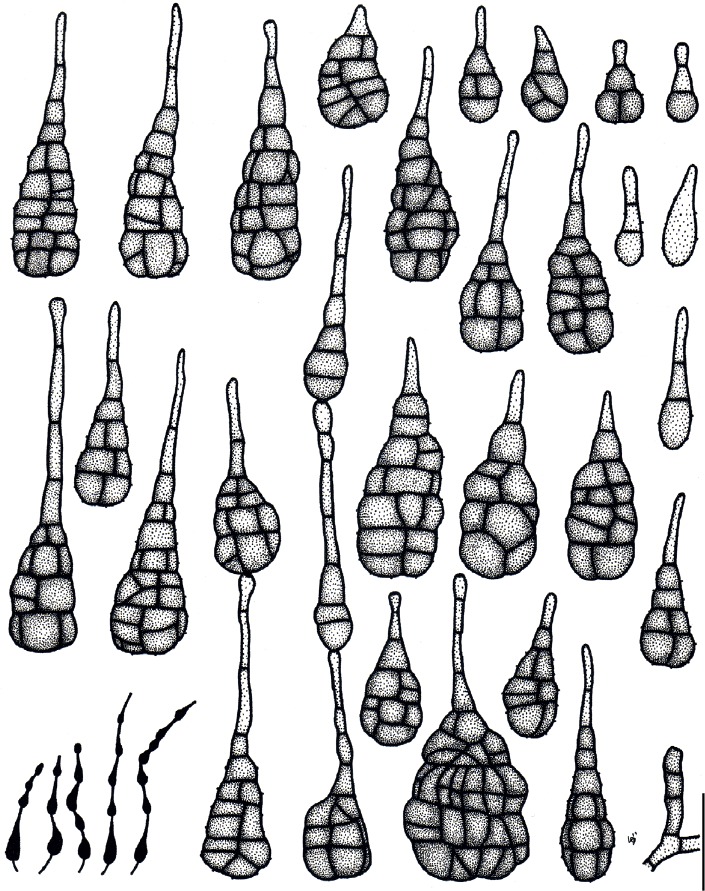
Illustrations of Alternaria triangularis (ex-holotype culture MAFF 246776). Morphology of conidia and conidiophores, and sporulation patterns (opaque) on V8 juice agar medium. Scale bar = 25 µm.
Fig. 47.
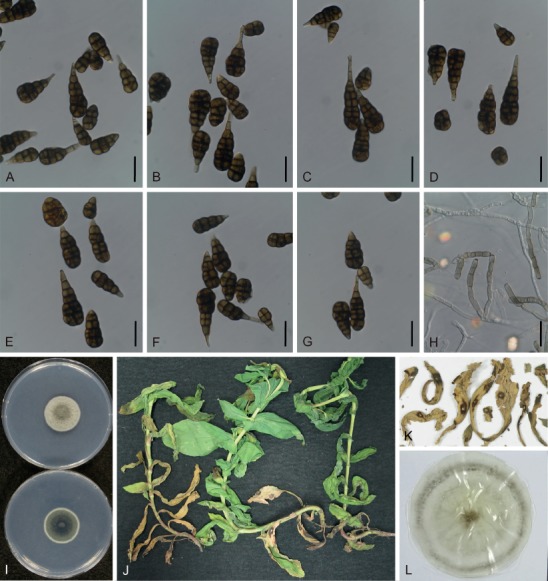
Morphological features of Japanese isolates of Alternaria triangularis (ex-holotype culture MAFF 246776). A–G. Conidia on V8 juice agar (V8) medium. H. Conidiophores on V8 medium. I. Culture on potato-dextrose agar medium (upper = surface, lower = reverse). J–K. Natural symptoms on Bupleurum. L. Dried culture specimen ex MAFF 246776 (holotype: TSN-F-85454). Scale bars (A–H) = 25 µm.
Etymology: Named after Latin “triangularis”, referring to the triangular shape of the conidia.
Diagnosis: The morphology of this species includes isosceles triangle-shaped conidia, comprising multi-celled bodies and elongated secondary conidiophores, and is quite unique among Apiaceae-pathogenic species. Phylogenetic analysis suggested that this species is a monotypic lineage sister to the sect. Sonchi and monotypic lineage A. brassicae. The species is also characterized by its host range, which is restricted to Bupleurum.
Leaf spots are circular, 4–10 mm diam, dark brown to black with a grayish eye at center, and are distinct at the border; leaf defoliation follows. On V8 medium, conidiophores short to moderately long, narrow, 16–59 × 4–6 µm. Conidia primarily in chains of 3–5, up to 8–9 (short to moderately long chains), lateral branches uncommon 5–7 d after incubation, pale brown to brown, long ovoid to obclavate, triangular to campanuloid in maturity, 15–93 µm in total length. Conidial bodies 14–53 × 6–33 µm, with 1–9 transverse septa and 0–11 (commonly in each unit, and sometimes complexed) longitudinal septa. The basal 1–2 units broadest, almost flattened bottoms, smooth to faintly rough. False beaks (secondary conidiophores) often elongated in 2–3 cells, up to 47 × 6 µm. On lesions, conidiophores 19–67 × 3–6 µm. Conidia 10–76 µm in total length; conidial bodies 10–45 × 5–23 µm, with 0–9 transverse septa and 0–9 longitudinal septa. False beaks up to 41 × 6 µm.
Typus: Japan, Kochi Prefecture, Konan, on leaves of Bupleurum rotundifolium, 9 Jan. 2004, J. Nishikawa (holotype TNS-F-85454) (a dried culture specimen ex MAFF 246776), isotypes MUMH 11669 and 11700, culture ex-holotype MAFF 246776, GenBank accession number ITS: LC440629, gapdh: LC482050, rpb2: LC476837, tef1: LC480255, Alt a 1: LC481641, act: LC481891.
Additional material examined: Japan, Shizuoka Prefecture, Kakegawa, on B. rotundifolium, 7 Jun. 2004, Y. Makizumi, living culture AC95.
Experimental host range: Selectively pathogenic to Bupleurum among members of the Apiaceae family, but weakly pathogenic or opportunistic to Angelica (Table 6).
Distribution: Only known from Japan.
Colony characteristics on PDA after 7 d at 25 °C: Slow-growing, reaching an average of 23.9 ± 0.9 mm diam; aerial hypha cottony, dense, grayish green to dark green, with white margins; reverse center dark green to black; sporulation sparse; diffusible pigment absent.
Sexual morph: Not observed.
DISCUSSION
During our survey of Alternaria species in Japan, we obtained and examined 85 isolates. Based on morphological observations, molecular phylogeny, and phenotyping with an experimental host range, 23 known species (including four species newly recorded from Japan) and three novel species were found. Moreover, phenotyping with an experimental host range not only helped determine each species boundary, but also revealed closely related and indistinguishable taxa of the examined Japanese species.
In the present study, five Japanese species with two formae speciales were clearly recognized in sect. Alternaria. Based on the conidial morphology of A. iridicola on holotype material, Simmons (2007) determined the taxonomic affinity of the species with small-spored species, namely within sect. Alternaria. Although Gannibal & Lawrence (2018a) suggested that Russian isolates had intermediate characteristics in both sect. Panax and Porri, conidial morphology of the examined Japanese isolates were identical to ex-type descriptions (Ellis & Everhart 1894, Simmons 2007), especially large conidia with long false beaks and catenate conidia were particularly diagnostic for a species in sect. Alternaria (Figs 22, 23). As the result of the multi-locus phylogeny, it was also revealed that this species clearly belongs to sect. Alternaria (Fig. 1). Since there are no living ex-type isolates, a dried-culture specimen (TNS-F-85452) was deposited as an epitype of A. iridicola. There are two related taxa, A. iridiaustralis and A. iridis, infecting Iris within the section. The results of the inoculation test with A. iridicola demonstrated that this species is not pathogenic to I. ensata (Table 8), which is a natural host of A. iridiaustralis (Luo et al. 2018). Therefore, these related species were distinguishable from A. iridicola based on host selectivity, as well as morphology and phylogeny. In addition, a novel species, A. cylindrica having selective pathogenicity to Petunia, was described, and some of the other species in this section (e.g., A. alstroemeriae and A. gaisen in the present study) also have selectivity within a host genus, species, or variety. Species in this section generally have distinct host specificity, and it is possible that potential host-selective toxin producers may be present.
In sect. Alternantherae, A. paragomphrenae was newly described in the present study. It was suggested that species in sect. Alternantherae were clearly differentiated in their pathogenicity to Amaranthaceae plants, reflecting their morphological and phylogenetic differences (Tables 3, 4; Fig. 1). Two species infecting Gomphrena, A. gomphrenae and A. paragomphrenae, were non-pathogenic to Amaranthoideae plants, including Amaranthus and Celosia, and were distinguishable from each other in pathogenicity to Alternanthera (Table 4). The remaining species had wide host ranges across the subfamilies in Amaranthaceae, and A. celosiicola was pathogenic to Amaranthoideae and Gomphrenoideae. In addition, it was considered that A. alternantherae and A. perpunctulata were conspecific, as they share a common original host (Alternanthera), are morphologically similar, and show high phylogenetic affinity (Zhao & Zhang 2005).
In sect. Brassicicola, Japanese isolates of A. brassicicola including the non-Brassica isolate MAFF 246773 were equally aggressive to a wide range of Brassicaceae hosts (Table 5). However, these isolates clustered in a well-supported single lineage together with ex-type isolates of A. mimicula, A. septorioides, and A. solidaccana, which were isolated from non-Brassica hosts – Solanum, Reseda, and soil, respectively (Fig. 1). Based on their host ranges within Brassicaceae, morphological similarity, and ubiquitousness of A. brassicicola (Simmons 2007, Farr & Rossman 2018), these three names were synonymized. Although multi-locus phylogeny resolved two subclades in this section with high BS and PP support values (Fig. 1), it was concluded that this section is a possible monotypic lineage recognized by the ITS phylogeny rather than the multi-locus phylogeny (Fig. 2).
Likewise, in sect. Japonicae, Japanese isolates of A. japonica were restricted within Brassicaceae (Table 5), and their conspecificity to A. nepalensis, the ex-type of which was isolated from Brassica sp., were supported by phylogenetic analyses conducted during the present study (Fig. 1). Because of its morphological similarity to the original description of A. nepalensis (Simmons 2007), this species was synonymized with A. japonica, and this section was typified as a monotypic lineage. It is interesting that during the evolution and differentiation of the genus Alternaria, three common pathogens that infect Brassicaceae (A. brassicae, A. brassicicola, and A. japonica) had almost no differences in their host ranges; nevertheless, they were distinctive in their conidial morphology and phylogenetic relationship to each other.
Alternaria cumini (sect. Eureka) and A. triangularis, which both infect Apiaceae, were morphologically and phylogenetically distinguishable from other species, including A. dauci (sect. Porri), and selectively pathogenic to each original host genus (Table 6). In sect. Radicina, the morphology and pathogenicity of A. petroselini were also easily distinguishable from those of related species, except A. selini (Nishikawa & Nakashima 2013). It was appropriate to synonymize A. selini with A. petroselini based on multi-locus phylogenic similarities. According to Park et al. (2008), two species pathogenic to Daucus, A. radicina and A. carotiincultae, were phylogenetically recognized as a distinct species based on their Alt a 1, tef1, and β-tubulin gene sequences, but not on their gapdh and rpb2 sequences (Woudenberg et al. 2013). Further studies will be required to resolve these species boundaries.
In sect. Gypsophilae, Japanese isolates and a representative isolate of A. nobilis clustered into a single lineage together with ex-type isolates of A. ellipsoidea and A. saponariae based on the ITS phylogeny (Fig. 2). However, the multi-locus phylogeny conducted in this and previous studies delimited a distinct subclade consisting of only Japanese isolates from some subclades of existing taxa (Fig. 1; Woudenberg et al. 2013, Gannibal 2019). Conidial morphology of Japanese isolates was identical with those of A. nobilis, but not clearly distinguishable from closely related species. This study could not examine pathological phenotyping with host range evaluations under the concept of integrated species recognition. Further studies are needed to resolve this contradiction and define the species boundaries of Dianthus pathogens in this section.
In sect. Panax, Japanese isolates and three representative isolates of A. panax clustered into a single lineage together with A. dendropanacis with strong BS and PP support. However, this clade was divided into three subclades with lower BS (ML and MP) support (Fig. 1), which were assigned to A. panax, A. araliae, and A. dendropanacis by Deng et al. (2015). Based on its conidial morphology, culture characteristics, and original host plants of Japanese isolates, all of these features overlapped with one another and were not identical to the definition presented by Deng et al. (2015). Although it needs to be verified by further studies such as cross-inoculation tests, it was appropriate that these taxa should be regarded as one species, A. panax, as suggested by the ITS phylogeny (Fig. 2).
In the present study five Japanese species were clearly recognized in sect. Porri. Morphological distinctions based on the color of their beaks was an especially effective diagnostic feature. Among these, colored beak species (A. cucumerina and A. zinniae) were not resolved in the ITS phylogeny (Fig. 2). However, host range has been generally applied as species criteria in this section (Neergaard 1945, Ellis 1971, Zhang 2003, Simmons 2007), and these two species were also well correlated with their hosts (Cucurbitaceae and Zinnia, respectively). As the result of the multi-locus phylogeny, the species boundary between these species was strongly supported, reflecting their host range (Fig. 1). In contrast, Woudenberg et al. (2014) phylogenetically differentiated A. porri from A. allii, and Japanese isolates clustered across the two species with lower BS and PP support (Fig. 1). However, it was affected only by the rpb2 phylogeny, and was not supported by other genes (ITS, gapdh, tef1, Alt a 1, and act) (data not shown). Based on the similarity of their conidial morphology and host range between both species, A. allii was rejected as a distinct species. It was also suggested that a multi-locus phylogeny was not always appropriate to resolve species boundaries in this section. This section is the largest, containing 63 species (Woudenberg et al. 2014), wherein phenotyping with both detailed morphological examinations and experimental host range are not sufficient. The ubiquitousness of A. porri, which was also shown in the present study, also demonstrated that integrated species recognition is strongly recommended to define the species boundaries of this section.
Thus far, we have discussed the utility of phenotyping based on experimental host ranges to distinguish closely related species. However, since the former Ulocladium species (especially for A. atra and A. chartarum) and one of the most frequent contaminants, A. alternata, were commonly established as saprophytic isolates, it is difficult to examine their taxonomy under the concept of integrated species recognition. Further approaches, such as secondary metabolite assays and inoculation tests to a few recorded susceptible hosts, are required to determine species boundaries among saprophytic species.
As a result of comprehensive inoculation tests, distinctive host selectivity was found along with the systematic ranks of each host plant, not only with genus but also subfamily, tribe, species, and variety, for most plant-pathogenic species of Alternaria. Moreover, phenotyping with experimental host ranges contributed to define species boundaries in Alternaria, supporting morphology and molecular phylogenetic data. This study also suggested that the ITS region is generally still effective with regard to DNA barcoding for the genus Alternaria, except for sect. Alternaria, and colored beak species in sect. Porri. It was concluded that integrated species recognition based on morphology, phylogeny, and pathogenicity helps elucidate species boundaries in the genus Alternaria, and will provide a practical, re-defined species concept for the genus.
ACKNOWLEDGEMENTS
We thank Dr. Hiromichi Horie, Hosei University, Dr. Toyozo Sato, National Institute of Agrobiological Sciences, Mr. Tsuyoshi Ono, Tokyo Metropolitan Agriculture and Forestry Research Center, and Dr. Keiko T. Natsuaki, Tokyo University of Agriculture, for sharing their specimens and isolates. Dr. Tsuyoshi Hosoya, National Museum of Nature and Science, also allowed us to examine specimens in TNS. The first author would like to express his gratitude to Dr. Yosuke Matsuda and Dr. Hiroki Matsui, Mie University, for taking the time to review and give comments on an earlier version of the manuscript. Mr. Kenji Takebayashi, Mr. Yoshiyuki Makizumi, Ms. Ayumi Kawagoe, and all the members of the Plant Pathology Division, Kakegawa Research Center, Sakata Seed Co., and Ms. Misato Takahashi, Mie University, are also gratefully acknowledged for their special support and encouragements. This study received financial support from the Institute for Fermentation, Osaka, Japan, and a JSPS KAKENHI grant (no. 17K07837) to CN.
Conflict of interest: The authors declare that no known conflicts of interests exists.
REFERENCES
- Abdel-Hafez SII. (1984). Mycoflora of bean, broad bean, lentil, lupine and pea seeds in Saudi Arabia. Mycopathologia 88: 45–49. [Google Scholar]
- Alonso R, Tiscornia S, Bettucci L. (2011). Fungal endophytes of needles and twigs from Pinus taeda and Pinus elliottii in Uruguay. Sydowia 63: 141–153. [Google Scholar]
- Atilano RA. (1983). A foliar blight of Ming aralia caused by Alternaria panax. Plant Disease 67: 224–226. [Google Scholar]
- Aveling TAS, Naude SP. (1992). First report of Alternaria porri on garlic in South Africa. Plant Disease 76: 643. [Google Scholar]
- Barkat EH, St J Hardy GE, Ren Y, et al. (2016). Fungal contaminants of stored wheat vary between Australian states. Australasian Plant Pathology 45: 621–628. [Google Scholar]
- Bassimba DDM, Mira JL, Baixauli C, et al. (2012). First report of Alternaria petroselini causing leaf blight of fennel in Spain. Plant Disease 96: 907. [DOI] [PubMed] [Google Scholar]
- Bassimba DDM, Mira JL, Vicent A. (2013). First report of Alternaria japonica causing black spot of turnip in Spain. Plant Disease 97: 1505. [DOI] [PubMed] [Google Scholar]
- Benjamin CR, Slot A. (1969). Fungi of Haiti. Sydowia 23: 125–163. [Google Scholar]
- Bettucci L, Alonso R, Fernandez LM. (1997). A comparative study of fungal populations in healthy and symptomatic twigs and seedlings of Eucalyptus globulus in Uruguay. Sydowia 49: 109–117. [Google Scholar]
- Blagojević J, Dolovac N, Ivanovic Z, et al. (2015). First report of horseradish leaf spot caused by Alternaria brassicae in Serbia. Plant Disease 99: 730. [Google Scholar]
- Boedo C, Benichou S, Berruyer R, et al. (2012). Evaluating aggressiveness and host range of Alternaria dauci in a controlled environment. Plant Pathology 61: 63–75. [Google Scholar]
- Bokura U. (1915). Handbook of plant disease management (in Japanese). Journal of Plant Protection (Tokyo) 2: 51–52. [Google Scholar]
- Bouckaert R, Heled J, Kühnert D, et al. (2014). BEAST 2: A Software platform for Bayesian evolutionary analysis. PLoS Computational Biology 10 (4): e1003537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso CR. (2014). Potato and tomato early blight: Molecular identification of Alternaria species, host range and epidemics. Ph.D. dissertation. Universidade Federal de Viçosa, Brazil. [Google Scholar]
- Caesar AJ, Lartey RT. (2009). First report of a leaf spot caused by Alternaria brassicae on the invasive weed Lepidium draba in North America. Plant Disease 93: 846. [DOI] [PubMed] [Google Scholar]
- Chaerani R, Groenewald R, Stam P, et al. (2007). Assessment of early blight (Alternaria solani) resistance in tomato using a droplet inoculation method. Journal of General Plant Pathology 73: 96–103. [Google Scholar]
- Chen W-q, Ntahimpera N, Morgan DP, et al. (2002). Mycoflora of Pistacia vera in the central valley, California. Mycotaxon 83: 147–158. [Google Scholar]
- Cho HS, Kim BR, Yu SH. (2001). Taxonomic studies on Alternaria in Korea. Mycobiology 29: 27–42. [Google Scholar]
- Cho JT, Moon BJ. (1980). The occurrence of strawberry black leaf spot caused by Alternaria alternata (Fr.) Keissler in Korea (in Korean). Korean Journal of Plant Protection 19: 221–226. [Google Scholar]
- Christias Ch, Hatzipapas P, Dara A, et al. (2001). Alternaria alternata, a new pathotype pathogenic to aphids. Biological Control 46: 105–124. [Google Scholar]
- Crous PW, Phillips AJL, Baxter AP. (2000). Phytopathogenic fungi from South Africa. University of Stellenbosch, Department of Plant Pathology Press. [Google Scholar]
- Cunnington JH, Minchinton EJ, Auer DPF, et al. (2007). First report of Alternaria petroselini sensu lato causing leaf blight on parsley in Australia. Plant Pathology 56: 723. [Google Scholar]
- De Hoog GS, Horré R. (2002). Molecular taxonomy of the Alternaria and Ulocladium species from humans and their identification in the routine laboratory. Mycoses 45: 259–276. [DOI] [PubMed] [Google Scholar]
- Deighton FC, MacGarvie QD. (1968). Alternaria longissima sp. nov. Mycological Papers 113: 1–15. [Google Scholar]
- Delgado G. (2011). Nicaraguan fungi: A checklist of Hyphomycetes. Mycotaxon 115: 534. [Google Scholar]
- Deng JX, Kim CS, Oh ES, et al. (2010). First report of foliar blight on Dendropanax morbifera caused by Alternaria panax. Mycobiology 38: 316–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng JX, Li MJ, Paul NC, et al. (2015). Alternaria species associated with araliaceous plants in Korea. Mycological Progress 14: 31. [Google Scholar]
- Deng JX, Paul NC, Park MS, et al. (2013). Molecular characterization, morphology, and pathogenicity of Alternaria panax from araliaceous plants in Korea. Mycological Progress 12: 383–396. [Google Scholar]
- Dingley JM. (1970). Records of fungi parasitic on plants in New Zealand 1966–68. New Zealand Journal of Agricultural Research 13: 325–337. [Google Scholar]
- Downs SH, Mitakakis TZ, Marks GB, et al. (2001). Clinical importance of Alternaria exposure in children. American Journal of Respiratory and Critical Care Medicine 164: 455–459. [DOI] [PubMed] [Google Scholar]
- Elliott JA. (1917). Taxonomic characters of the genera Alternaria and Macrosporium. American Journal of Botany 4: 439–476. [Google Scholar]
- Ellis MB. (1971). Dematiaceous Hyphomycetes. Commonwealth Mycological Institute, Kew, Surrey, England. Ellis MB (1976). More dematiaceous Hyphomycetes. Commonwealth Mycological Institute, Kew, Surrey, England. [Google Scholar]
- Ellis JB, Everhart BM. (1894). New species of fungi from various localities. Proceedings of the Academy of Natural Sciences of Philadelphia 46: 322–384. [Google Scholar]
- Enjoji S. (1931). Two new diseases of cineraria I. Ring spot disease (in Japanese). Journal of Plant Protection (Tokyo) 18: 428–432. [Google Scholar]
- Esfahani MN. (2018). Identification of Ulocladium atrum causing potato leaf blight in Iran. Phytopathologia Mediterranea 57: 112–114. [Google Scholar]
- Farr DF, O’Neill NR, van Berkum PB. (2000). Morphological and molecular studies on Dendryphion penicillatum and Pleospora papaveraceae, pathogens of Papaver somniferum. Mycologia 92: 145–153. [Google Scholar]
- Farr DF, Rossman AY. (2018). Fungal Databases. U.S. National Fungus Collections, ARS, USDA. Retrieved August 24, 2018, from https://nt.ars-grin.gov/fungaldatabases/. [Google Scholar]
- Farr ML, Stevenson JA. (1963). Eine erganzungsliste bolivianischer pilze. Sydowia 17: 37–69. [Google Scholar]
- Farrar JJ, Pryor BM, Davis RM. (2004). Alternaria diseases of carrot. Plant Disease 88: 776–784. [DOI] [PubMed] [Google Scholar]
- Felsenstein J. (1985). Confidence limits on phylogenies: An approach using the bootstrap. Evolution 39: 783–791. [DOI] [PubMed] [Google Scholar]
- Gaetan S, Madia M. (2005). First report of gray leaf spot caused by Alternaria brassicae on canola in Argentina. Plant Disease 89: 207. [DOI] [PubMed] [Google Scholar]
- Garibaldi A, Bertetti D, Poli A, et al. (2013). First report of leaf spot of Saponaria officinalis caused by Alternaria nobilis in Italy. Plant Disease 97: 424. [DOI] [PubMed] [Google Scholar]
- Garibaldi A, Gilardi G, Gullino ML. (2004). First report of Alternaria leaf blight of Aralia japonica caused by Alternaria panax in Europe. Plant Disease 88: 82. [DOI] [PubMed] [Google Scholar]
- Gasich EL, Gannibal PB, Berestetskiy AO, et al. (2013). Taxonomically significant characters of Crivellia papaveracea and Brachycladium papaveris, pathogens of poppy, revealed in Russia and Ukraine (in Russian). Mikologiya i Fitopatologiya 47: 240–251. [Google Scholar]
- Gannibal PB. (2011). Alternaria cucumerina causing leaf spot of pumpkin newly reported in North Caucasus (Russia). New Disease Reports 23: 36. [Google Scholar]
- Gannibal PB. (2018). Distribution of Alternaria species among sections. 4. Species formerly assigned to genus Nimbya. Mycotaxon 133: 37–44. [Google Scholar]
- Gannibal PB. (2019). New species and new findings in Russia of Alternaria sect. Gypsophilae. Mikologiya I Fitopatologiya 53: 10–16. [Google Scholar]
- Gannibal PB, Gasich EL. (2009). Causal agents of the alternariosis of cruciferous plants in Russia: Species composition, geography and ecology (in Russian). Mikologiya i Fitopatologiya 43: 447–456. [Google Scholar]
- Gannibal PB, Lawrence DP. (2018a). Distribution Alternaria species among sections. 5. Species producing conidia with many longitudinal septa. Mycotaxon 133: 285–291. [Google Scholar]
- Gannibal PB, Lawrence DP. (2018b). Distribution of Alternaria species among sections. 6. Species formerly assigned to genus Ulocladium. Mycotaxon 133: 293–299. [Google Scholar]
- Geng Y, Li Z, Xia LY, et al. (2014). Characterization and phylogenetic analysis of the mating-type loci in the asexual ascomycete genus Ulocladium. Mycologia 106: 649–665. [DOI] [PubMed] [Google Scholar]
- Goto K. (1927). Disease notes in Hiroshima (in Japanese). Journal of Plant Protection (Tokyo) 14: 454–463. [Google Scholar]
- Groves JW, Skolko AJ. (1944). Notes on seed-borne fungi II: Alternaria. Canadian Journal of Research, Section C, Botanical Sciences 22: 217–253. [Google Scholar]
- Guo LD, Xu L, Zheng WH, et al. (2004). Genetic variation of Alternaria alternata, an endophytic fungus isolated from Pinus tabulaeformis as determined by random amplified microsatellites (RAMS). Fungal Diversity 16: 53–65. [Google Scholar]
- Hall BH, Hitch CJ, Oxspring EA, et al. (2007). Leek diseases in Australia. Australasian Plant Pathology 36: 383–388. [Google Scholar]
- Hayashi N, Yamamoto M, Tsuge T, et al. (1992). Comparison of parasitic fitness between two pathotypes of Alternaria alternata to Japanese pear. Japanese Journal of Phytopathology 58: 734–740. [Google Scholar]
- Heredia G, Sierra MA, Portales MJ. (1995). Conidial fungi from leaf litter in a mesophilic cloud forest of Veracruz, Mexico. Mycotaxon 55: 473–490. [Google Scholar]
- Hirayama S, Imura J. (1941). Identification of the leaf blight pathogen on poppy [Abstract] (in Japanese). Japanese Journal of Phytopathology 10: 338–339. [Google Scholar]
- Huang LH, Hanlin RT. (1975). Fungi occurring in freshly harvested and in-market pecans. Mycologia 67: 689–700. [PubMed] [Google Scholar]
- Hyun IH, Chang SY, Lee MY, et al. (2012). Seed-borne Brachycladium penicillatum intercepted under plant quarantine inspection in Korea. Mycobiology 40: 205–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichinose R, Nishikawa J, Morita K, et al. (2015). New leaf spot diseases of Datura spp. caused by Alternaria crassa (in Japanese). Annual Report of the Kanto-Tosan Plant Protection Society 62: 83–86. [Google Scholar]
- Imai S. (1914). Diseases of ornamental flowers (in Japanese). Journal of Plant Protection (Tokyo) 1: 75–78. [Google Scholar]
- Inderbitzin P, Shoemaker RA, O’Neill NR, et al. (2006). Systematics and mating systems of two fungal pathogens of opium poppy: the heterothallic Crivellia papaveracea with a Brachycladium penicillatum asexual state and a homothallic species with Brachycladium papaveris asexual state. Canadian Journal of Botany 84: 1304–1326. [Google Scholar]
- Infantino A, Giambattista GD, Pucci N, et al. (2009). First report of Alternaria petroselini on fennel in Italy. New Disease Reports 19: 26. [Google Scholar]
- Jasalavich CA, Morales VM, Pelcher LE, et al. (1995). Comparison of nuclear ribosomal DNA sequences from Alternaria species pathogenic to crucifers. Mycological Research 99: 604–614. [Google Scholar]
- Joly P. (1964). Le genre Alternaria: Recherches physiologiques, biologiques et systématiques. Encyclopédie Mycologique 33. Paul Lechevalier, Paris. [Google Scholar]
- Katoh K, Rozewicki J, Yamada KD. (2017). MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Briefings in Bioinformatics 10.1093/bib/bbx108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katumoto K. (2010). List of Fungi Recorded in Japan. The Kanto branch of the Mycological Society of Japan, Funabashi, Japan. [Google Scholar]
- Kirk PM, Cannon PF, Minter DW, et al. (2008). Ainsworth & Bisby’s dictionary of the fungi, Edn 10. CAB International, Wallingford. [Google Scholar]
- Koike ST. (1996). Japanese mustard, tah tsai, and red mustard as hosts of Alternaria brassicae in California. Plant Disease 80: 822. [Google Scholar]
- Koike ST, Henderson DH. (1998). Purple blotch, caused by Alternaria porri, on leek transplants in California. Plant Disease 82: 710. [DOI] [PubMed] [Google Scholar]
- Koike ST, Molinar RH. (1997). Daikon (Raphanus sativus cv. longipinnatus) as a host of Alternaria brassicae in California. Plant Disease 81: 1094. [DOI] [PubMed] [Google Scholar]
- Kowalski T, Andruch K. (2012). Mycobiota in needles of Abies alba with and without symptoms of Herpotrichia needle browning. Forest Pathology 42: 183–190. [Google Scholar]
- Kozlov A, Darriba D, Flouri T, et al. (2018). RAxML-NG: A fast, scalable, and user-friendly tool for maximum likelihood phylogenetic inference. bioRxiv 447110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranz J. (1963). Fungi collected in the Republic of Guinea II: Collections from the Kindia area in 1962. Sydowia 17: 174–185. [Google Scholar]
- Kumar S, Stecher G, Tamura K. (2016). MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Molecular Biology and Evolution 33: 1870–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence DP, Park MS, Pryor BM. (2012). Nimbya and Embellisia revisited, with nov. comb for Alternaria celosiae and A. perpunctulata. Mycological Progress 11: 799–815. [Google Scholar]
- Lawrence DP, Gannibal PB, Peever TL, et al. (2013). The sections of Alternaria: formalizing species-group concepts. Mycologia 105: 530–546. [DOI] [PubMed] [Google Scholar]
- Lawrence DP, Gannibal PB, Dugan FM, et al. (2014). Characterization of Alternaria isolates from the infectoria species-group and a new taxon from Arrhenatherum, Pseudoalternaria arrhenatheria sp. nov. Mycological Progress 13: 257–276. [Google Scholar]
- Lawrence DP, Rotondo F, Gannibal PB. (2016). Biodiversity and taxonomy of the pleomorphic genus Alternaria. Mycological Progress 15: 3. [Google Scholar]
- Liu YH, Zhang CQ, Xu BC. (2013). First report of leaf blight in Chinese hickory (Carya cathayensis) caused by Alternaria petroselini in China. Plant Disease 97: 1253. [DOI] [PubMed] [Google Scholar]
- Lopes MC, Martins VC. (2008). Fungal plant pathogens in Portugal: Alternaria dauci. Revista Iberoamericana de Micología 25: 254–256. [PubMed] [Google Scholar]
- Lunghini D, Granito VM, Di Lonardo DP, et al. (2013). Fungal diversity of saprotrophic litter fungi in a Mediterranean maquis environment. Mycologia 105: 1499–1515. [DOI] [PubMed] [Google Scholar]
- Luo H, Tao YQ, Fan XY, et al. (2018). Identification and characterization of Alternaria iridiaustralis causing leaf spot on Iris ensata in China. Mycobiology 46: 168–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagano K. (1920). Ring spot disease of pear (in Japanese). Journal of the Japanese Horticultural Society 32: 16–19. [Google Scholar]
- Nakashima C, Araki I, Kobayashi T. (2011). Addition and reexamination of Japanese species belonging to the genus Cercospora and allied genera. X: Newly recorded species from Japan (5). Mycoscience 52: 253–259. [Google Scholar]
- Neergaard P. (1945). Danish species of Alternaria and Stemphylium: Taxonomy, parasitism, economical significance. Einar Munksgaard Publishers, Copenhagen. [Google Scholar]
- Nishikawa J, Kobayashi T, Shirata K, et al. (2006). Seedborne fungi detected on stored solanaceous berry seeds and their biological activities. Journal of General Plant Pathology 72: 305–313. [Google Scholar]
- Nishikawa J, Nakashima C. (2013). Taxonomic characterization and experimental host ranges of four newly recorded species of Alternaria from Japan. Journal of Phytopathology 161: 604–616. [Google Scholar]
- Nishikawa J, Nakashima C. (2015). Morphological variation and experimental host range of Alternaria cinerariae. Mycoscience 56: 141–149. [Google Scholar]
- Nishikawa J, Nakashima C. (2019). Morphological and molecular characterization of the strawberry black leaf spot pathogen referred to as the strawberry pathotype of Alternaria alternata. Mycoscience 60: 1–9. [Google Scholar]
- Nishimura S. (1980). Host-specific toxins from Alternaria alternata: Problems and prospects. Proceedings of the Japan Academy, Series B 56: 362–366. [Google Scholar]
- Ono T. (2004). Occurrence of Alternaria leaf spot in Ming aralia and geraniumleaf aralia caused by Alternaria panax on Ogasawara (Bonin) Islands (in Japanese). Annual Report of the Kanto-Tosan Plant Protection Society 51: 67–69. [Google Scholar]
- Ozkilinc H, Rotondo F, Pryor BM, et al. (2018). Contrasting species boundaries between sections Alternaria and Porri of the genus Alternaria. Plant Pathology 67: 303–314. [Google Scholar]
- Park MS, Romanoski CE, Pryor BM. (2008). A re-examination of the phylogenetic relationship between the causal agents of carrot black rot, Alternaria radicina and Alternaria carotiincultae. Mycologia 100: 511–527. [DOI] [PubMed] [Google Scholar]
- Phillips DJ, Mackey B, Ellis WR, et al. (1979). Occurrence and interaction of Aspergillus flavus with other fungi on almonds. Phytopathology 69: 829–831. [Google Scholar]
- Poudel B, Zhang S. (2018). First report of Alternaria leaf spot on cilantro (Coriandrum sativum) caused by Alternaria dauci in the United States. Plant Disease 102: 822. [Google Scholar]
- Pryor BM, Asma M. (2007). First report of seedling damping-off of fennel caused by Alternaria petroselini in the Netherlands. Plant Disease 91: 1688. [DOI] [PubMed] [Google Scholar]
- Pryor BM, Gilbertson RL. (2000). Molecular phylogenetic relationships amongst Alternaria species and related fungi based upon analysis of nuclear ITS and mt SSU rDNA sequences. Mycological Research 104: 1312–1321. [Google Scholar]
- Pryor BM, Gilbertson RL. (2002). Relationships and taxonomic status of Alternaria radicina, A. carotiincultae, and A. petroselini based upon morphological, biochemical, and molecular characteristics. Mycologia 94: 49–61. [PubMed] [Google Scholar]
- Pomella AWV, Barreto RW, Charudattan R. (2007). Nimbya alternantherae a potential biocontrol agent for alligatorweed, Alternanthera philoxeroides. BioControl 52: 271–288. [Google Scholar]
- Rambaut A, Drummond AJ, Xie D, et al. (2018). Posterior summarisation in Bayesian phylogenetics using Tracer 1.7. Systematic Biology 67: 901–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao VG. (1969). The genus Alternaria - from India. Nova Hedwigia 17: 219–258. [Google Scholar]
- Rathod S. (2012). Seed borne Alternaria species: A review. Current Botany 3: 21–23. [Google Scholar]
- Rayner RW. (1970). A mycological colour chart. Commonwealth Mycological Institute; Kew, Surrey, England. [Google Scholar]
- Ren XX, Zhang GZ, Dai WA. (2012). First report of damping-off caused by Alternaria japonica on Chinese cabbage seedlings in China. Plant Disease 96: 1378. [DOI] [PubMed] [Google Scholar]
- Richardson MJ. (1990). An annotated list of seed-borne diseases, Edn 4 International Seed Testing Association, Zurich. [Google Scholar]
- Rossman AY, Lu KC. (1980). Filamentous fungi associated with leaf surfaces of red alder and Douglas-fir seedlings in western Oregon. Mycotaxon 10: 369–371. [Google Scholar]
- Rossmann SN, Cernoch PL, Davis JR. (1996). Dematiaceous fungi are an increasing cause of human disease. Clinical Infectious Diseases 22: 73–80. [DOI] [PubMed] [Google Scholar]
- Runa F, Park MS, Pryor BM. (2009). Ulocladium systematics revisited: Phylogeny and taxonomic status. Mycological Progress 8: 35–42. [Google Scholar]
- Sato T. (2015). Fungi isolated from rotten bean sprouts and its spoiled ingredients (in Japanese). MAFF Microorganism Genetic Resources Manual 37: 1–19. [Google Scholar]
- Sato T, Aoki M, Aoki T, et al. (2014). Fungi isolated from spoiled bean sprouts in Japan. The Japan International Research Center for Agricultural Sciences 48: 317–329. [Google Scholar]
- Sawada K. (1944). Descriptive catalogue of the Formosan fungi. Part X. Report of the Department of Agriculture, Government Research Institute, Formosa 87: 1–96. [Google Scholar]
- Seifert K, Morgan-Jones G, Gams W, et al. (2011). The genera of Hyphomycetes. CBS Biodiversity Series 9. Centraalbureau voor Schimmelcultures, Utrecht. [Google Scholar]
- Sharma TR, Tewari JP. (1998). RAPD analysis of three Alternaria species pathogenic to crucifers. Mycological Research 102: 807–814. [Google Scholar]
- Shimazaki H. (1930). Disease on Iris japonica caused by Macrosporium sp. (in Japanese). Journal of Plant Protection (Tokyo) 17: 459–463. [Google Scholar]
- Shtienberg D. (1994). Achene blemish syndrome: a new disease of sunflower in Israel. Plant Disease 78: 1112–1116. [Google Scholar]
- Siciliano I, Gilardi G, Ortu G, et al. (2017). Identification and characterization of Alternaria species causing leaf spot on cabbage, cauliflower, wild and cultivated rocket by using molecular and morphological features and mycotoxin production. European Journal of Plant Pathology 149: 401–413. [Google Scholar]
- Simmons EG. (1967). Typification of Alternaria, Stemphylium, and Ulocladium. Mycologia 59: 67–92. [PubMed] [Google Scholar]
- Simmons EG. (1971). Helminthosporium allii as type of a new genus. Mycologia 63: 380–386. [Google Scholar]
- Simmons EG. (1986). Alternaria themes and variations (22–26). Pleospora / Stemphylium and Lewia / Alternaria. Mycotaxon 25: 287–308. [Google Scholar]
- Simmons EG. (1989). Macrospora Fuckel (Pleosporales) and related anamorphs. Sydowia 41: 314–329. [Google Scholar]
- Simmons EG. (1995a). Alternaria chronology and catalogue raisonné: part I, 1796–1871. Mycotaxon 55: 1–53. [Google Scholar]
- Simmons EG. (1995b). Alternaria themes and variations (112–144). Mycotaxon 55: 55–163. [Google Scholar]
- Simmons EG. (1997). Alternaria themes and variations (151–223). Mycotaxon 65: 1–92. [Google Scholar]
- Simmons EG. (2002). Alternaria themes and variations (287–304). Species on Caryophyllaceae. Mycotaxon 82: 1–40. [Google Scholar]
- Simmons EG. (2004). Novel dematiaceous Hyphomycetes. Studies in Mycology 50: 109–118. [Google Scholar]
- Simmons EG. (2007). Alternaria: An identification manual. CBS Biodiversity Series 6. Centraalbureau voor Schimmelcultures, Utrecht. [Google Scholar]
- Soylu S, Kurt S, Soylu EM, et al. (2004). First report of Alternaria leaf blight caused by Alternaria dauci on carrot in Turkey. New Disease Reports 10: 3. [Google Scholar]
- Stamatakis A. (2014). RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30: 1312–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starast M, Galynskaya N, Jõgar K, et al. (2009). Blueberry diseases survey in Estonia. Agronomy Research 7 (Special issue I): 511–516. [Google Scholar]
- Stevenson JA. (1975). Fungi of Puerto Rico and the American Virgin Islands. Contributions from the Reed Herbarium. Baltimore, MD 23: 743. [Google Scholar]
- Su XJ, Yu H, Zhou T, et al. (2005). First report of Alternaria raphani causing black patches on Chinese radish during postharvest storage in Canada. Plant Disease 89: 1015. [DOI] [PubMed] [Google Scholar]
- Swofford DL. (2003). PAUP*. Phylogenetic analysis using parsimony (*and other methods). Version 4.0b10. Sinauer Associates, Sunderland, Massachusetts. [Google Scholar]
- Takano K. (2005). Alternaria blight, a new disease of petunia (Petunia hybrida Vilm) caused by Alternaria longissima Deighton & MacGravie [Abstract] (in Japanese). Japanese Journal of Phytopathology 71: 73. [Google Scholar]
- Tanabe AS. (2011). Kakusan4 and aminosan: Two programs for comparing nonpartitioned, proportional and separate models for combined molecular phylogenetic analyses of multilocus sequence data. Molecular Ecology Resources 11: 914–921. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Harada Y. (2003). Pleosporales in Japan (1): the genus Lophiostoma. Mycoscience 44: 85–96. [Google Scholar]
- Tao WC, Zhang W, Yan JY, et al. (2014) A new Alternaria species from grapevine in China. Mycological Progress 13: 999. [Google Scholar]
- Togashi K. (1926). On a new species of Alternaria causing a leafspot disease of Gomphrena globosa L. Bulletin of the Imperial college of Agriculture and Forestry, Morioka, Japan. 9: 1–16. [Google Scholar]
- Tohyama A. (1993). Isolation and identification of fungi 35: Genus Alternaria (in Japanese). Journal of Antibacterial and Antifungal Agents 21: 107–115. [Google Scholar]
- Tohyama A, Tsuda M. (1990). Alternaria on cruciferous plants 1. Identity of Alternaria japonica and A. raphani. Transactions of the Mycological Society of Japan 31: 501–509. [Google Scholar]
- Tokumasu S, Aoki T, Oberwinkler F. (1994). Fungal succession on pine needles in Germany. Mycoscience 35: 29–37. [Google Scholar]
- Uchida JY, Aragaki M, Yoshimura MA. (1984). Alternaria leaf spots of Brassaia actinophylla, Dizygotheca elegantissima, and Tupidanthus calyptratus. Plant Disease 68: 447–449. [Google Scholar]
- van de Wouw AP, Idnurm A, Davidson JA, et al. (2016). Fungal disease of canola in Australia: Identification of trends, threats and potential therapies. Australasian Plant Pathology 45: 415–423. [Google Scholar]
- Vannini A, Vettraino AM. (2000). Ulocladium chartarum as the causal agent of a leaf necrosis on Quercus pubescens. Forest Pathology 30: 297–360. [Google Scholar]
- Wada H, Cavanni P, Bugiani R, et al. (1996). Occurrence of the strawberry pathotype of Alternaria alternata in Italy. Plant Disease 80: 372–374. [Google Scholar]
- Watanabe T, Uematsu S, Sato Y. (1986). Fungus isolates from Japanese black and red pine seeds with some taxonomical notes. Bulletin of the Government Forestry Experiment Station 336: 1–18. [Google Scholar]
- Watanabe T, Sato Y. (1988). Fungi isolated from Japanese cedar and cypress seeds in Japan. Japanese Journal of Mycology 29: 143–150. [Google Scholar]
- Watanabe Y, Umekawa M. (1977). Black spot of strawberry caused by Alternaria sp. [Abstract] (in Japanese). Japanese Journal of Phytopathology 43: 82. [Google Scholar]
- Wang Y, Geng Y, Ma J, et al. (2011). Sinomyces: a new genus of anamorphic Pleosporaceae. Fungal Biology 115: 188–195. [DOI] [PubMed] [Google Scholar]
- Wicker EF, Yokota S. (1982). Fungi associated with blister rust cankers on Pinus strobus and P. pumila in Japan. Transactions of the Mycological Society of Japan 23: 143–148. [Google Scholar]
- Woudenberg JHC, Groenewald JZ, Binder M, et al. (2013). Alternaria redefined. Studies in Mycology 75: 171–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woudenberg JHC, Seidl MF, Groenewald JZ, et al. (2015). Alternaria section Alternaria: Species, formae speciales or pathotypes? Studies in Mycology 82: 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woudenberg JHC, Truter M, Groenewald JZ, et al. (2014). Large-spored Alternaria pathogens in section Porri disentangled. Studies in Mycology 79: 1–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue F, Zhang XG. (2007). Ulocladium capsicuma, a new species identified by morphological and molecular phylogenetic data. Sydowia. 59: 161–178. [Google Scholar]
- Yamagishi N, Nishikawa J, Oshima Y, et al. (2009). Black spot disease of alstroemeria caused by Alternaria alstroemeriae in Japan. Journal of General Plant Pathology 75: 401–403. [Google Scholar]
- Ye YF, Fu G, Jiang N, et al. (2013). First report of leaf spot caused by Alternaria porri on velvet bean (Mucuna pruriens) in China. Plant Disease 97: 141. [DOI] [PubMed] [Google Scholar]
- Yoshii H. (1929a). Purple blotch on bunching onion caused by Macrosporium porri Ellis (in Japanese). Journal of Plant Protection (Tokyo) 16: 466–472. [Google Scholar]
- Yoshii H. (1929b). Alternaria black rot on carrot (in Japanese). Journal of Plant Protection (Tokyo) 16: 660–664. [Google Scholar]
- Yoshii H. (1933). On the pathogenic organism of the leaf spot disease of Gomphrena globosa (in Japanese). Japanese Journal of Phytopathology 2: 513–519. [Google Scholar]
- Yoshii H. (1941). Alternaria species on cruciferous plants (in Japanese). Journal of Plant Protection (Tokyo) 28: 14–18. [Google Scholar]
- You MP, Simoneau P, Dongo A, et al. (2005). First report of an Alternaria leaf spot caused by Alternaria brassicae on Crambe abyssinicia in Australia. Plant Disease 89: 430. [DOI] [PubMed] [Google Scholar]
- Yu SH. (2001). Korean species of Alternaria and Stemphylium. National Institute of Agricultural Science and Technology, Suwon, Korea. [Google Scholar]
- Zarandi DM, Sharzei A. (2015). First report of lemon verbena leaf spot caused by Ulocladium chartarum in Iran. Plant Disease 99: 1186. [Google Scholar]
- Zhang TY. (2003). Alternaria. Flora fungorum sinicorum. vol. 16 Science Press, Beijing. [Google Scholar]
- Zhang M, Tsukiboshi T, Okabe I. (2009). First report of Alternaria panax causing leaf spot of Aralia cordata in Japan. Plant Disease 93: 1215. [DOI] [PubMed] [Google Scholar]
- Zhao GZ, Zhang TY. (2005). Notes on dictyosporous Hyphomycetes from China VII. The genus Nimbya. Fungal Diversity 19: 201–215. [Google Scholar]


