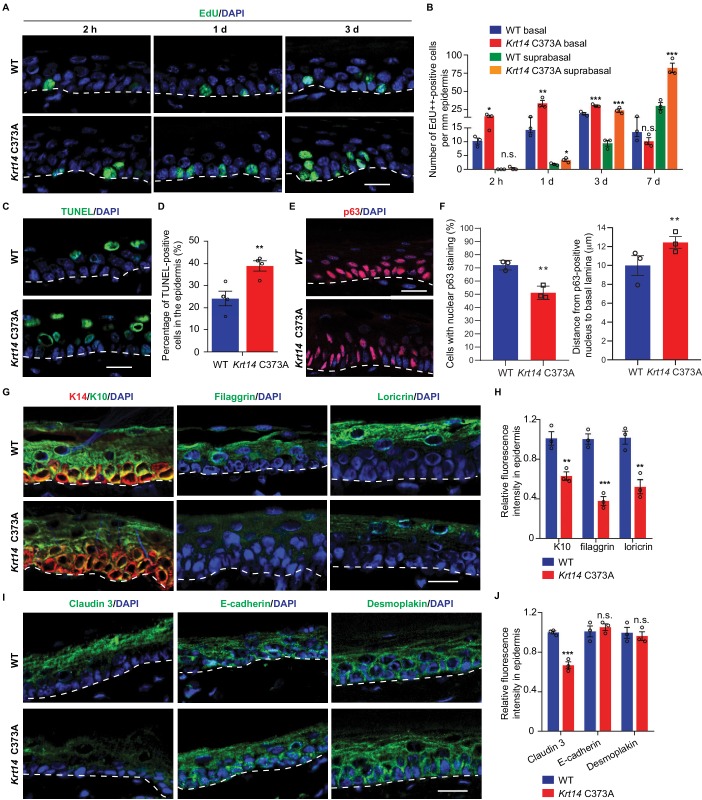
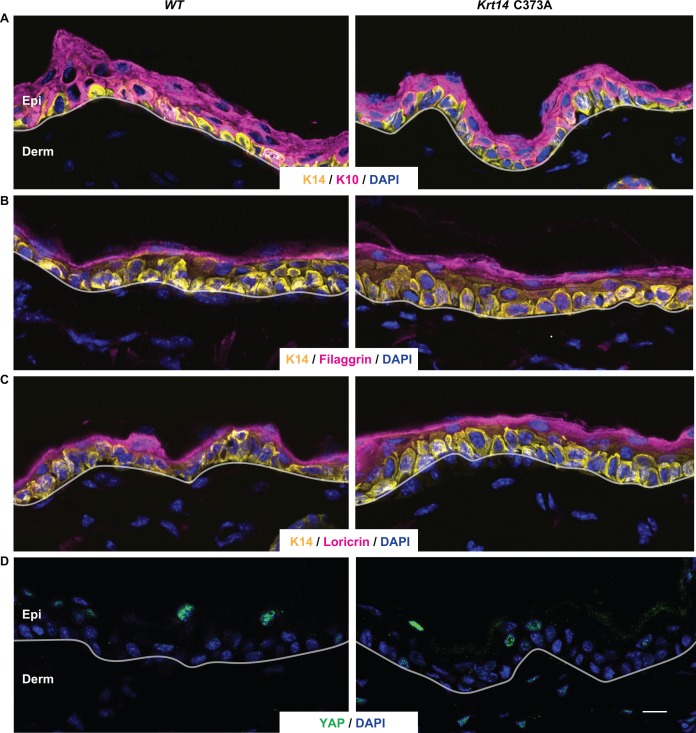
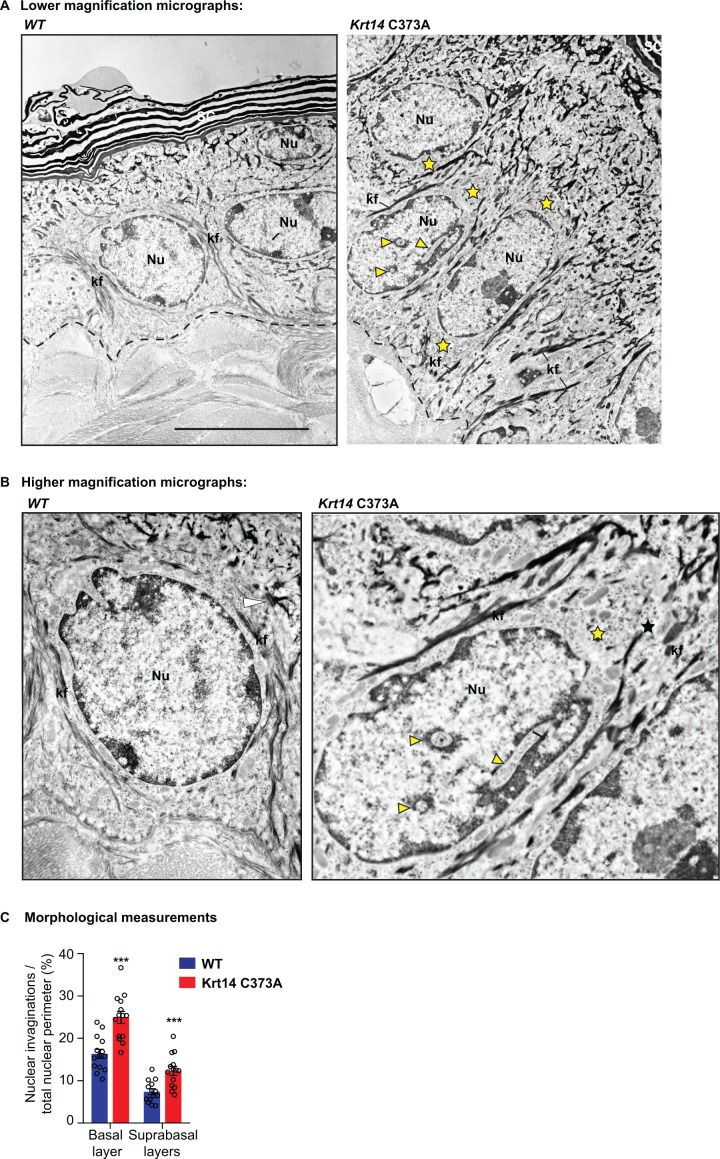
Figure 3. Altered tissue homeostasis and dysregulated keratinocyte differentiation in Krt14 C373A skin.
(A) Indirect immunofluorescence for Edu in tail skin section from WT and Krt14 C373A at 2 hr, 1 d, and 3 d after treatment with thymidine analog EdU. Nuclei as stained with DAPI (blue). (B) Quantification of number of EdU-positive nuclei in basal and suprabasal layers per mm of epidermis. N = 3 replicates for each sample. (C) TUNEL staining in tail epidermis of young adult WT and Krt14 C373A mice. D. Quantification of TUNEL-positive cells shown in frame c. N = 4 mice per sample. E. Indirect immunofluorescence for p63 in tail skin section from WT and Krt14 C373A tail skin. Dashed lines depict the dermo-epidermal interface. (F) Quantification of the number of p63-positive nuclei per mm of epidermis (left) and their distance from the basal lamina (right). N = 3 replicates for each sample. (G) Indirect immunofluorescence for K14 (green), K10 (red), filaggrin, and loricrin from tail skin sections of WT and Krt14 C373A mice. (H) Quantification of relative fluorescence intensity of data shown in frame g, normalized to WT. N = 3 mice per sample. (I) Indirect immunofluorescence for claudin 3, E-cadherin and desmoplakin in tail skin sections from WT and Krt14 C373A mice. (J) Quantitation of relative fluorescence intensity in g. N = 3 mice per sample. In a, c, e, g, and I, nuclei are stained with DAPI (blue), and dashed lines depict the dermo-epidermal interface. Scale bars, 20 µm. Data in b, d, f, h and g represent mean ± SEM. Student’s t test: *p<0.05; **p<0.01; ***p<0.005; n.s., no difference.