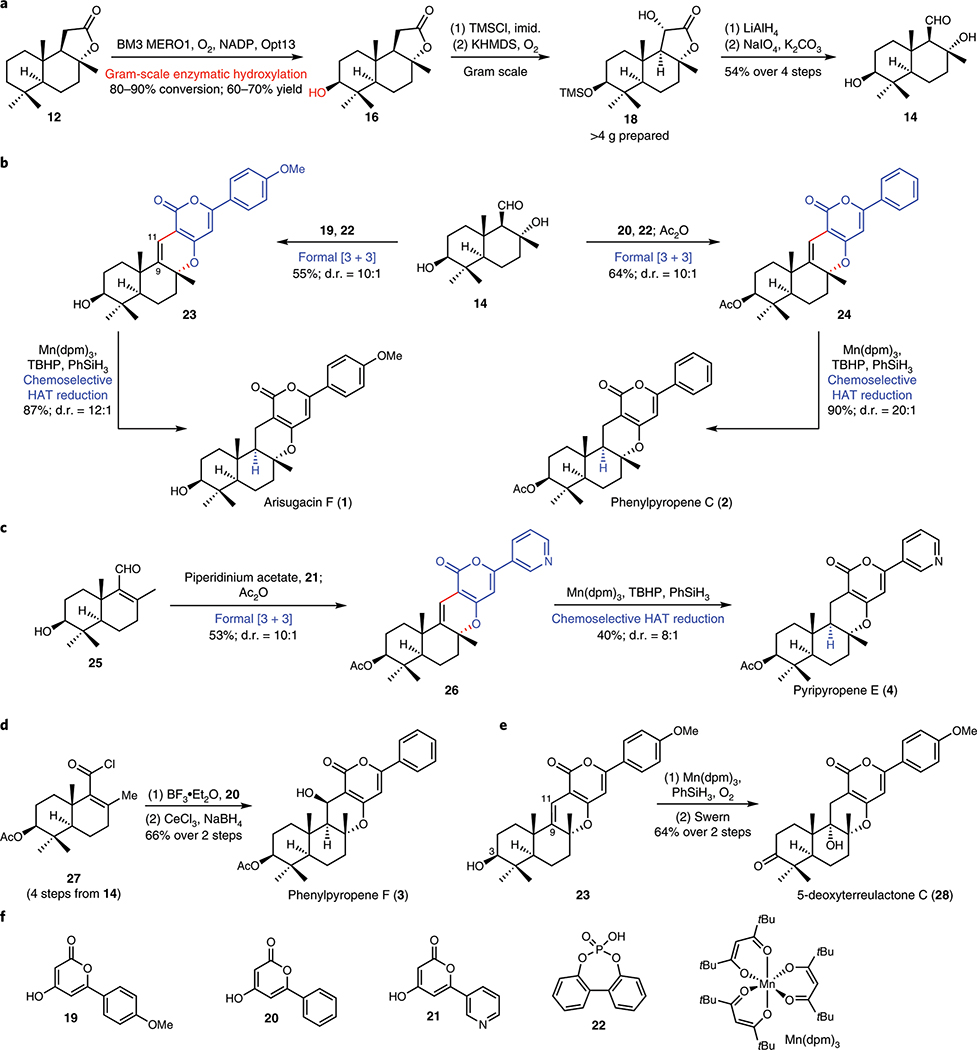
Figure 3. Modular chemoenzymatic synthesis of α-pyrone meroterpenoids.
a. Gram-scale biocatalytic hydroxylation of sclareolide (12), followed by oxidative degradation of its lactone moiety, enabled rapid access to key aldehyde 14. b. Compound 14 was converted to arisugacin F (1) and phenylpyropene C (2) via [3 + 3] coupling to append the appropriate pyrone units and HAT hydrogenation to selectively reduce the C9–C11 olefin. c. Compound 14 was dehydrated to enal 25, which in turn could be converted to pyripyropene E (4) via a similar [3 + 3] coupling/HAT hydrogenation sequence. 1, 2 and 4 are obtained in 7–8 steps from 12; biocatalytic installation of C3–OH and the use of radical-based logic for reduction at C9 are important transformations in their syntheses. d. Acid chloride 27, available from 14 in 4 steps, was converted to phenylpyropene F (3) via [3 + 3] coupling, followed by Luche reduction of the C11 ketone. e. A chemoselective Mukaiyama hydration of the C9–C11 olefin of 23 completed the synthesis of 5-deoxyterreulactone C (28). f. Chemical structures of reagents used in the transformations shown in this figure. NADP, nicotinamide adenine dinucleotide phosphate; TMSCl, trimethylsilyl chloride; imid., imidazole; KHMDS, potassium bis(trimethylsilyl)amide; dpm, 2,2,6,6-tetramethyl-3,5-heptanedionato; TBHP, tert-butyl hydrogen peroxide.