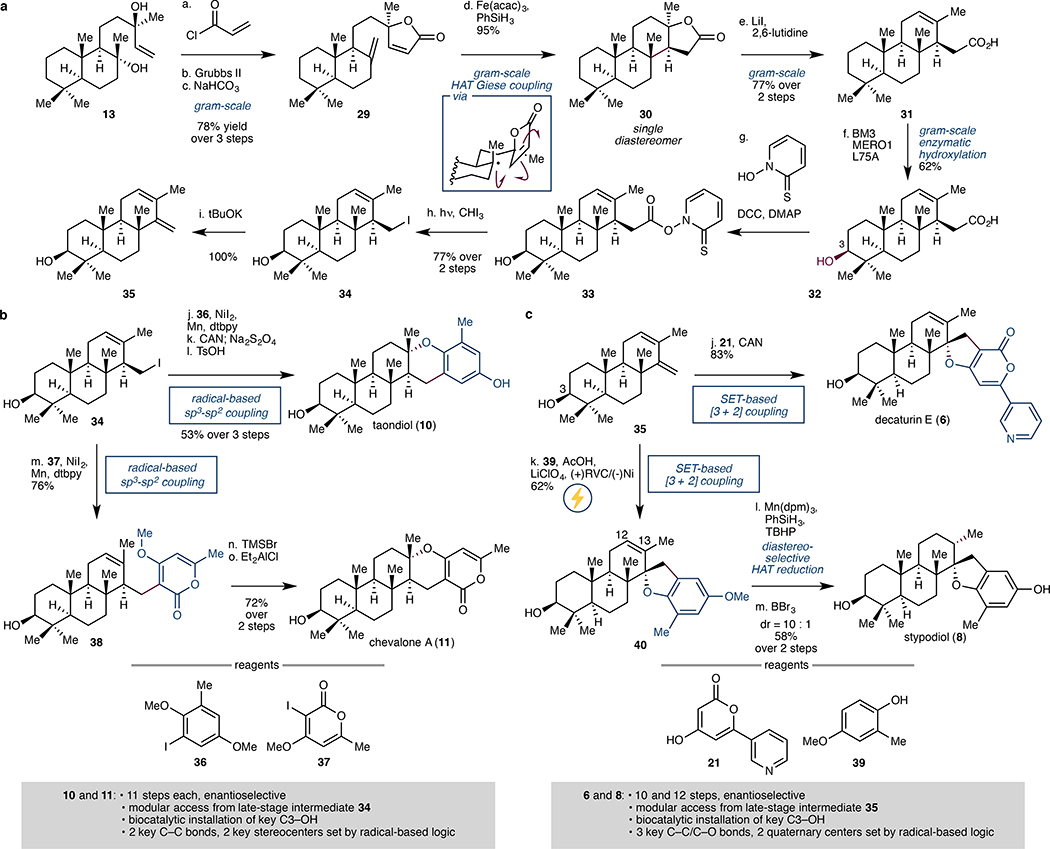
Figure 4. Modular chemoenzymatic synthesis of diterpenic meroterpenoids.
a. Building blocks 34 and 35 were synthesised from sclareol (13) in 8 and 9 steps, respectively. Key transformations in the sequence include the use of HAT-based intramolecular Giese coupling to form the C-ring with complete diastereoselectivity and the use of biocatalytic hydroxylation with an engineered P450BM3 to selectively install the C3 alcohol. b. Taondiol (10) and chevalone A (11) were synthesised in modular fashion from late-stage intermediate 34 via Ni-catalysed cross coupling with aryl iodides 36 and 37, respectively. Both 10 and 11 are accessed enantioselectively in 11 steps from 13; the full route involves biocatalytic installation of the key C3–OH and the formation of 2 key C–C bonds and 2 key stereocentres using radical-based logic. c. Decaturin E (6) and stypodiol (8) were synthesised in modular fashion from late-stage intermediate 35 via SET-based [3 + 2] coupling with pyrone 21 and phenol 39, respectively. Enantioselective formation of 6 and 8 occurs in 10 and 12 steps, respectively, from 13. Key transformations include the biocatalytic installation of C3–OH and the formation of 3 C–C/C–O bonds and 2 quaternary centres using radical-based logic. Acac, acetyl acetone; DCC, N,N’-dicyclohexylcarbodiimide; DMAP, 4-dimethylaminopyridine; dtbpy, 4,4´-di-tert-butyl-2,2´-dipyridyl; CAN, ceric ammonium nitrate; SET, single electron transfer; TMSBr, trimethylsilyl bromide; RVC, reticulated vitreous carbon.