Fig. 2. Safety and Pharmacokinetics.
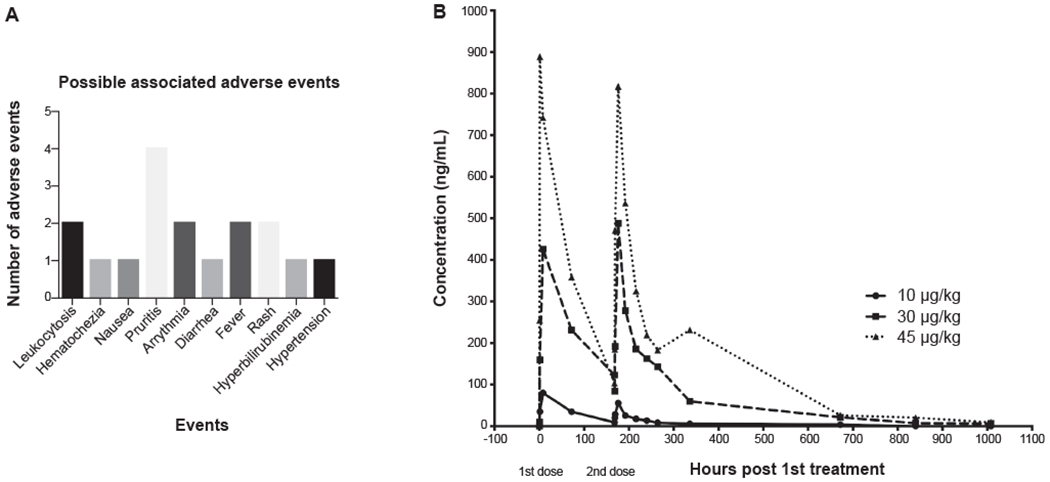
(A) Safety and tolerability of F-652 as determined by absence of significant adverse events (SAE). There were no SAE related to the study drug leading to discontinuation. (B) Pharmacokinetics showing half-life of F-652. The half-life of F-652 following the first dose was 61-85 h.
