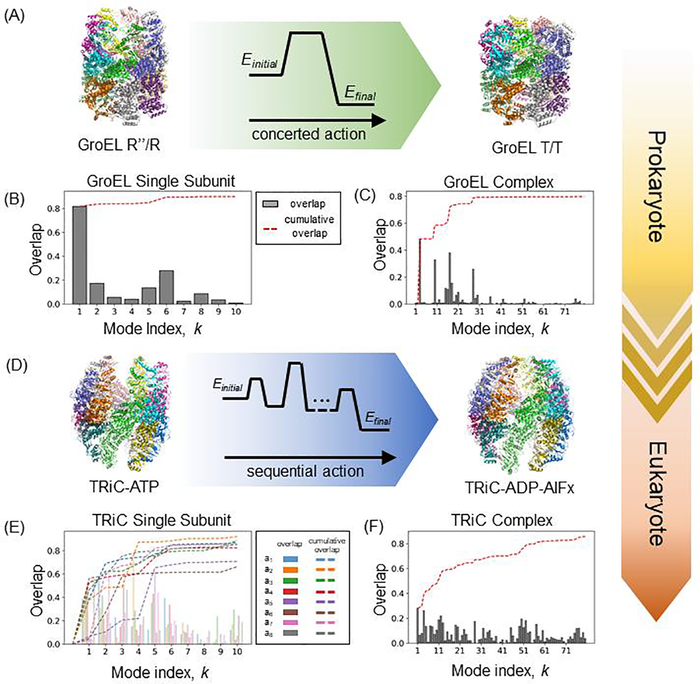
Figure 2. Differential modes of action of prokaryotic and eukaryotic chaperonins.
(A and D) The transition of GroEL from cis-ring GroES-bound (R”) state (PDB id: 1gru) to apo (T) state (PDB id: 1gr5) follows a concerted mode of action, which surmounts a single energy barrier. Whereas the transition of TRiC/CCT from ATP-bound, open state (PDB id: 4a0v) to an ADP-P-bound, closed state (PDB id: 4a0w) follows a sequential mode of action, which involves several possibly smaller energy barriers. Co-chaperonin GroES is not included for the purpose of direct comparison between the two states of GroEL. (B) Overlaps between the ten softest modes predicted by ENM to be accessible to a single subunit of GroEL in the R form, and the experimentally observed deformation undergone by the subunit during its transition to the T form. The bars display the correlation cosines for each mode, and the red curve displays the cumulative overlap (summed over consecutive modes starting from mode k = 1. Panel (C) displays the behavior of softest 80 modes accessible to the entire complex. The cumulative contribution of ~ 30 soft modes is sufficient to reach a correlation cosine of ~ 0.80 with the experimentally observed transition between the two endpoints shown in panel (A). (D-F) Counterparts of the respective panels (A) -(C) shown for TRiC/CCT (mammalian chaperonin). CCT rings are each hetero-octameric, hence eight overlap curves and bar plots corresponding to each of the (sequentially different) subunits are displayed in (E). Overall the passage between the two end points necessitates a larger ensemble of modes of motions, compared to the bacterial chaperonin, suggesting a more controlled/restrained allosteric machinery evolutionarily endowed by sequence/structure divergence.