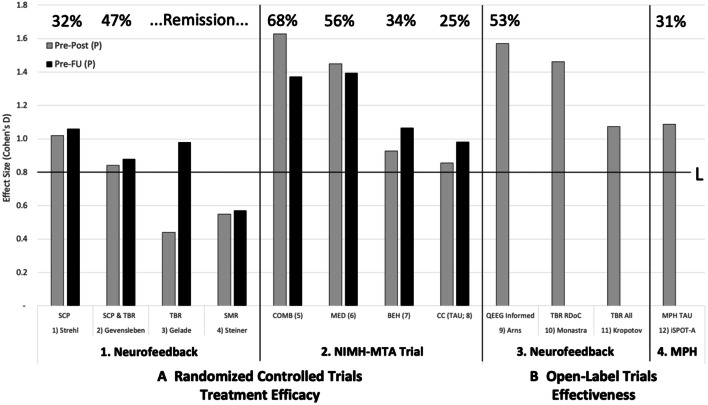
Fig. 1.
The landscape of ADHD treatments with pre-post treatment effect sizes (Cohen’s D; ES) for parent rated overall ADHD symptom improvement (grey, Pre-Post) and from pre-treatment to follow-up (black, Pre-FU), with indicators illustrating large ES (L: D > 0.8) and remission rates listed on top (top line; no data on remission rates was available for studies (3), (4) (10) and (11)). On the left the results for efficacy are depicted and on the right for effectiveness, separated in 1. Neurofeedback RCT’s, 2. NIMH-MTA treatment arms (Combined treatment (COMB), Medication only (MED), Multicomponent Behaviour Therapy (BEH) and Community Care—treatment as usual (CC (TAU)), 3. Neurofeedback open label trials and 4. Methylphenidate (MPH) open label data from the iSPOT-A study. Note the consistent increases in ES for neurofeedback studies from pre-treatment to follow-up, with the opposite trend for the MTA medication arms, indicating a strengthening of effects for neurofeedback over time, without additional treatment. The open label trials also demonstrate the benefit obtained in clinical settings is overall similar or better, relative to clinical trials, whereas the effects of open-label MPH tend to be lower relative to the MTA results. This demonstrates results of neurofeedback translate well into clinical practice (‘effectiveness’ based on standard protocols)