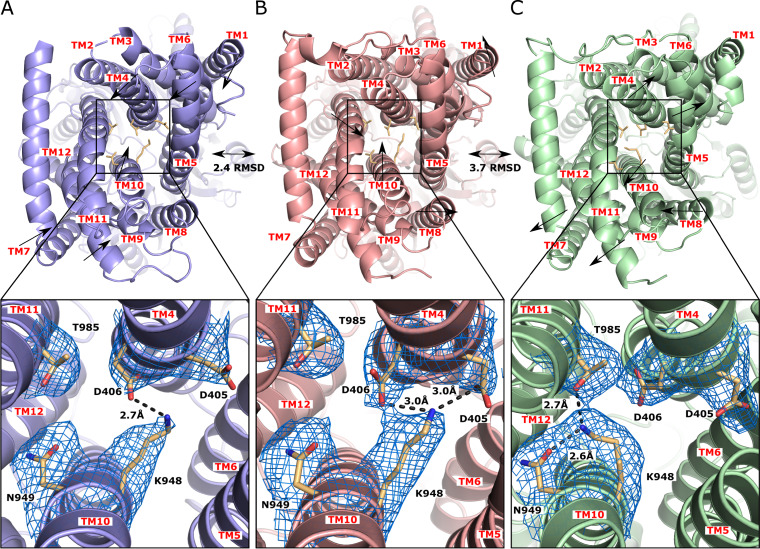
FIG 4.
The proton-relay network of the MtrDCR103 multidrug efflux pump. (A) The “access” state of MtrDCR103. In this conformational state, the “proton sweeper” K948 forms a hydrogen bond with D406. The densities of residue side chains (D405, D406, K948, N949, and T985), which form the proton-relay network, are in blue meshes. (B) The “binding” state of MtrDCR103. In this conformation, K948 forms hydrogen bonds with both D405 and D406. (C) The “extrusion” state of MtrDCR103. In this conformation, K948 forms hydrogen bonds with N949 and T985. Superimposition of the structures of the access and binding states results in an RMSD of 2.4 Å, whereas the superimposition between the binding and extrusion states gives rise to an RMSD of 3.7 Å.