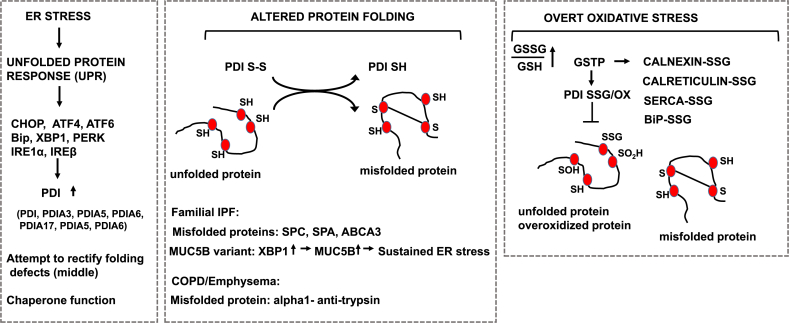
Fig. 3.
Disregulation of the Unfolded Protein Response (UPR) and protein disulfide isomerase in settings of chronic lung diseases. Left: Schematic overview of the link between ER stress, the resultant unfolded proteins response, and the effector pathways that are increased in chronic lung diseases. Middle: Illustrations of misfolded proteins relevant to IPF (SPC, SPA, ABCA3) or COPD (α1 anti-trypsin). Also illustrated is MUC5B which has been linked to ER stress in settings of familial IPF. Observed increases in PDI in chronic lung diseases may be to rectify the burden of ER stress and/or misfolded proteins. Right: In settings of overt oxidative stress, the function of PDI, and other ER proteins shown, may be compromised through oxidations and/or other modifications, allowing misfolded and/or overoxidized proteins to accumulate. Note that thus far, data to support this scenario was obtained in cell lines and/or settings of overt oxidative stress. Relevance of these putative events to chronic lung disease will require detailed analyses of human tissue specimens. We refer the reader to the body of text for detailed descriptions.