Abstract
OBJECTIVE:
Turkey is one of the latest countries that COVID-19 disease was reported, with the first case on March 11, 2020, and since then, Istanbul became the epicenter of the pandemic in Turkey. Here, we reveal sequences of the virus isolated from three different patients with various clinical presentations.
METHODS:
Nasopharyngeal swab specimens of the patients were tested positive for the COVID-19 by qRT-PCR. Viral RNA extraction was performed from the same swab samples. Amplicon based libraries were prepared and sequenced using the Illumina NextSeq platform. Raw sequencing data were processed for variant calling and generating near-complete genome sequences. All three genomes were evaluated and compared with other worldwide isolates.
RESULTS:
The patients showed various clinics (an asymptomatic patient, patient with mild disease, and with severe pulmonary infiltration). Amplicon-based next-generation sequencing approach successfully applied to generate near-complete genomes with an average depth of 2.616. All three viral genomes carried the D614G variant (G clade according to GISAID classification) with implications for the origin of a spread first through China to Europe then to Istanbul.
CONCLUSION:
Here, we report the viral genomes circulating in Istanbul for the first time. Further sequencing of the virus isolates may enable us to understand variations in disease presentation and association with viral factors if there is any. In addition, the sequencing of more viral genomes will delineate the spread of disease and will guide and ease the necessary measures taken to stem the spread of the novel coronavirus.
Keywords: COVID-19, SARS-CoV-2, whole-genome sequencing
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), causing coronavirus disease 2019 (COVID-19), was first detected in Wuhan, China, around mid-December 2019. Following the emergence of the outbreak to other countries in a short period, the World Health Organization declared COVID-19 as a pandemic on 11 March 2020. As of May 2020, more than 3.5 million COVID-19 patients all around the world were reported, and nearly 250 thousand deaths arose from the SARS-CoV-2 pandemic [1].
The novel SARS-CoV-2 belongs to the Betacoronavirus genus of the Coronaviridae family, which are single-stranded RNA viruses [2]. Genetic similarity analyses revealed that the bat coronavirus RaTG13 showed the highest similarity (96%) to SARS-CoV-2, and it was suggested that the zoonotic origin might include bats [3, 4]. Coronaviruses were thought to result in seasonal mild respiratory illness in humans until the epidemics of the SARS-CoV in 2003 and MERS-CoV in 2012 [5, 6].
As the number of COVID-19 cases increases worldwide, new symptoms have been reported, such as nausea, diarrhea, skin rash, and loss of taste and/or smell [7, 8]. In addition to the symptomatic patients, initial studies showed that a considerable amount of COVID-19 patients are asymptomatic (18–30%) or have mild symptoms [9–11], whereas hospitalization and mortality rates increase with patient’s age [12]. The severity of the infection is related to the age of the patient and the presence of additional chronic diseases, such as hypertension, diabetes, and cancer [13]. Besides challenges on detecting evidence on virulence changes of such a pathogen that is spreading very fast, there is not any solid report highlighting a mutation that affects the biological features of the virus.
The entrance of coronaviruses into the host cell is maintained by spike glycoprotein (encoded by S gene). The S1 subunit of SARS-CoV spike protein contains Receptor Binding Protein, which plays an essential role in the recognition of angiotensin-converting enzyme 2 (ACE2) [14]. The novel SARS-CoV-2 virus has a similar surface spike protein sharing 76% sequence identity with SARS-CoV [15]. The binding affinity of spike protein to ACE2 is important for virulence, and it has been shown that spike protein of the novel SARS-CoV-2 binds to ACE2 with a much higher affinity [16]. It can be hypothesized that a particular mutation in the spike protein may lead to conformational variations affecting the virulence.
Due to the lack of polymerase proofreading activity, RNA viruses have a relatively high mutation rate and, thus capable to become resistant to drugs and escape from host immunity. As mutations accumulate, they may result in alterations in virulence, transmission capacity, infectivity, and pathogenicity of the viruses [17]. Although SARS-CoV-2 has a lower mutation rate than expected [18], real-time tracking of the virus isolates in populations may help epidemiological understanding of the disease and early detection of important mutational or recombination events.
The first case of COVID-19 in Turkey was reported on 11 March 2020, much later than the virus had spread to European countries. As of April 1, the Ministry of Health of Turkey announced that COVID-19 had reached all over Turkey, exhibiting the highest spread in Istanbul. The first full-length SARS-CoV-2 genome in Turkey was isolated from a patient in Kayseri province and released on 13 April 2020. Herein, we analyzed full-length SARS-CoV-2 genomes from three patients in Istanbul together with their clinical findings.
MATERIALS AND METHODS
Sample Collection: Nasopharyngeal swabs were collected from unrelated patients and tested for SARS-CoV-2 presence as a standard care protocol for routine diagnosis in Umraniye Training and Research Hospital (UEAH), Istanbul. Three patients whose tests positive for SARS-CoV-2 with qRT-PCR testing were included in this study. This study was approved by the ethics committee of the Umraniye Training and Research Hospital (B.10.1.TKH.4.34.H.GP.0.01/95) and a written informed consent was obtained from all participating patients.
Viral RNA Extraction: Viral RNA was extracted from 200 µl of nasopharyngeal swab samples with High Pure Viral RNA Kit (Roche Life Science) according to the manufacturer’s protocol. Extracted RNA was eluted in 45 µl of DNase/RNase-free water. Total RNA concentration was determined with Qubit 4.0 Fluorometer using Qubit RNA HS Assay Kit (Thermo Fisher Scientific Inc.) and RNA quality was evaluated at 260/280 nm and 260/230 nm ratios using Nanodrop 2000. RNA samples were stored at −80 °C until further processing.
Viral Genome Sequencing and Data Analysis: Sequencing libraries were generated using the CleanPlex SARS-CoV-2 Library Preparation Kit (Paragon Genomics Inc.) following the manufacturer’s instructions. As input material, 50 ng of extracted RNA was used for each sample. Briefly, reverse transcription of RNA was performed; the viral genome was amplified with multiplex PCRs followed by indexing PCR to add adapters and sample-specific barcode sequences. DNA clean-up steps were performed with Agencourt AMPure XP beads (Beckman Coulter Inc.) when required to maximize the recovery of fragments. The quantity and quality of the final libraries were assessed using a Qubit dsDNA HS Assay Kit with Qubit 4.0 Fluorometer (Thermo Fisher Scientific Inc.) and an Agilent Bioanalyzer 2100 with High Sensitivity DNA Chips (Agilent Technologies Inc.) following manufacturers’ protocols before sequencing. Sequencing was performed in the joint Genomic Laboratory (GLAB) of UEAH and Istanbul Technical University [19] using Illumina NextSeq500 instrument with paired-end 150 bp chemistry. Raw demultiplexed sequencing data were further processed to call variants and generate consensus genome sequences. First, the quality check of raw sequencing data was performed using the FASTQC program [20] and adapter sequences were trimmed from reads using cutadapt [21]. Processed reads were aligned to the reference SARS-CoV-2 genome (NC_045512.2) using bwa-mem [22]. Further, primer sequences were trimmed from aligned bam files. Variant calling and generating consensus sequences were performed using Samtools [23] and iVar [24]. Finally, all detected variants were checked manually to detect the presence of any sequencing errors, if any. Viral genome sequences were examined phylogenetically together with world-wide isolates using Nextstrain [25, 26].
RESULTS
Clinical Presentation of the Patients
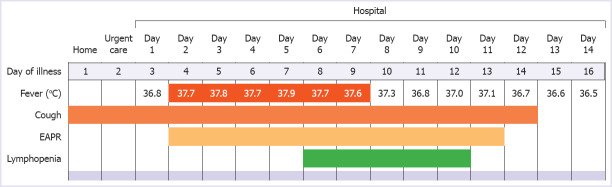
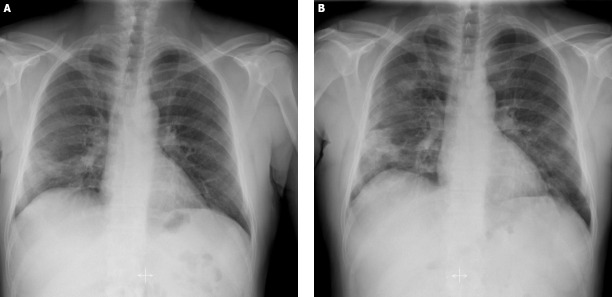
Patient COV-8: COV-8 was a 51-year-old man, and he presented to the emergency department clinic in our hospital with a 2-day history of cough. He did not mention fever or dyspnea. He had no known contact with a COVID-19 positive patient. He was an ex-smoker with a 50 packs-year history and had a medical history of diabetes mellitus and hypertension. The physical examination in the emergency department revealed a body temperature of 36.8°C, blood pressure of 120/70 mm Hg, the pulse of 100 beats per minute, respiratory rate of 20 breaths per minute, and oxygen saturation of 97% while the patient was breathing ambient air. Lung auscultation was normal. The remaining physical examination findings were unremarkable or normal. Nasopharyngeal and oropharyngeal swab specimens were collected and sent for real-time PCR (RT-PCR) assay for SARS-CoV-2. The patient complied with the possible case definition of COVID-19, which was stated in the COVID-19 Guide of the Ministry of Health of Turkey [27]. Therefore, thorax computed tomography (CT), in line with COVID-19 Guide [27], was performed and reported as containing typical findings of COVID pneumonia with mild involvement. Hospitalization was decided by considering the thorax CT result and the patient’s comorbid diseases. Azithromycin and hydroxychloroquine treatment combinations were started. On the first day of hospitalization, vital signs were in the normal range, and the general clinical condition was good. Viral RT-PCR analysis of the nasopharyngeal swab reported as positive. On the second and third day, subfebrile body temperature fluctuated between 37.7°C and 37.8°C were detected (Figure 1). Besides, there was an increase in the frequency of cough, and rales were detected during lung auscultation. An increase in infiltrations was also detected on the chest radiograph, as well as the increase in acute phase reactants (Figure 2). Close follow-up was continued by adding ceftriaxone to the treatment. On the 6th day, the subfebrile fever persisted, the patient reported fatigue. Oxygen saturation was <90% while inhaling in the room air. We observed that the serum acute phase reactants increased and lymphopenia appeared in the complete blood count test. In the thorax CT on the 6th day, we also detected progression compared to the previous CT scan. Due to the above-mentioned findings in favor of progression, favipiravir treatment was started. Twenty-four hours after the start of favipiravir, the patient’s body temperature returned to normal range. Through 7 and 14 days of the hospitalization, the patient’s clinical findings and laboratory values gradually improved. The physical examination revealed a body temperature of 36.5°C, blood pressure of 125/70 mm Hg, the pulse of 98 beats per minute, respiratory rate of 20 breaths per minute, and oxygen saturation of 98% while the patient was breathing ambient air. The remainder of the examination was normal. Viral RT-PCR analysis of the control nasopharyngeal swab on the 14th day reported as negative. He was discharged from the hospital to revisit for control two weeks later.
FIGURE 1.
Symptoms, maximum body temperatures, and laboratory findings of COV-8 according to the day of illness and day of hospitalization.
FIGURE 2.
Posteroanterior chest radiographs of COV-8 on the first (A) and the third (B) days of hospital stay (3rd and 5th day of illness).
Patient COV-12: COV-12 was a 49-year-old man and he presented to the COVID-19 clinic in our hospital with a history COVID-19 positive patient contact. The patient did not have any complaints. He was a healthy nonsmoker. The physical examination revealed a body temperature of 36.0°C, blood pressure of 130/70 mm Hg, the pulse of 100 beats per minute, respiratory rate of 18 breaths per minute, and oxygen saturation of 98% while the patient was breathing ambient air. Lung auscultation was normal, as well as the thorax CT. Complete blood count and serum acute phase reactants were in the normal range. Azithromycin and hydroxychloroquine treatment combinations were started, and the patient was isolated at home. The remaining physical examination findings were unremarkable or normal. The control viral RT-PCR test for SARS-CoV-2 was reported as negative.
Patient COV-13: COV-13 was a 29-year-old woman; she presented to the urgent care clinic with a 5-day history of cough, sore throat, fever, loss of taste and smell. She was a nonsmoker and reported no comorbid disease. The physical examination revealed a body temperature of 36.2°C, blood pressure of 140/75 mm Hg, the pulse of 96 beats per minute, respiratory rate of 20 breaths per minute, and oxygen saturation of 96%. The remaining physical examination findings were unremarkable or normal. Thorax CT revealed widespread patchy ground-glass opacities in both lungs. Complete blood count and serum acute phase reactants were in the normal range. She was hospitalized and started on azithromycin and hydroxychloroquine treatments. During the hospital stay, the patient did not have a fever and did not develop respiratory distress; oxygen saturation ranged between 96–97%. The treatment was completed in five days, and she was discharged to come for control one week later. After one week, the control examination of the patient was completely normal, for both vital signs and clinical findings. Control viral RT-PCR analysis was also reported as negative.
Viral Genome Analysis
Raw sequencing data consist of 600676, 390806 and 283036 paired-end reads for samples isolated from patients COV-8, COV-12 and COV-13, respectively. Nearly all reads were mapped to the 29.903 bp reference genome with a mean ratio of 99.02% (±0.85), resulting in an average 2.616±1.011 depth of coverage. Identified variants of three SARS-CoV-2 isolates are given in Table 1. Since all three isolates have a D614G variant in spike glycoprotein, they belong to G clade based on GISAID classification. COV-8 and COV-12 had ten, and COV-13 had nine bp changes compared to the reference genome (NC_045512.2). Distinguishably, most of the single nucleotide variants were C to T conversion.
TABLE 1.
Identified mutations in three SARS-CoV-2 isolates from Istanbul
| Sample | Nucleotide position | Nucleotide change (Ref/Alt) | Variant type | Amino acid position | Amino acid change (Ref/Alt) | Gene/Region |
|---|---|---|---|---|---|---|
| COV-8 | 241 | C/T | non-coding | NA | NA | 5’ UTR |
| 2113 | C/T | synonymous | 616 | I/I | orf1ab | |
| 2997 | C/T | missense | 911 | S/F | orf1ab | |
| 3037 | C/T | synonymous | 924 | F/F | orf1ab | |
| 7765 | C/T | synonymous | 2500 | S/S | orf1ab | |
| 14408 | C/T | missense | 314 | P/L | orf1ab | |
| 17690 | C/T | missense | 1408 | S/L | orf1ab | |
| 18877 | C/T | synonymous | 1804 | L/L | orf1ab | |
| 23403 | A/G | missense | 614 | D/G | S | |
| 25563 | G/T | missense | 57 | Q/H | orf3a | |
| COV-12 | 241 | C/T | non-coding | NA | NA | 5’ UTR |
| 2113 | C/T | synonymous | 616 | I/I | orf1ab | |
| 3037 | C/T | synonymous | 924 | F/F | orf1ab | |
| 7765 | C/T | synonymous | 2500 | S/S | orf1ab | |
| 14408 | C/T | missense | 314 | P/L | orf1ab | |
| 17690 | C/T | missense | 1408 | S/L | orf1ab | |
| 18877 | C/T | synonymous | 1804 | L/L | orf1ab | |
| 21452 | G/T | missense | 2662 | G/V | orf1ab | |
| 23403 | A/G | missense | 614 | D/G | S | |
| 25563 | G/T | missense | 57 | Q/H | orf3a | |
| COV-13 | 241 | C/T | non-coding | NA | NA | 5’ UTR |
| 3037 | C/T | synonymous | 924 | F/F | orf1ab | |
| 11083 | G/T | missense | 3606 | L/F | orf1ab | |
| 12809 | C/T | missense | 4182 | L/F | orf1ab | |
| 14408 | C/T | missense | 314 | P/L | orf1ab | |
| 23403 | A/G | missense | 614 | D/G | S | |
| 28881-28882 | GG/AA | missense | 203 | R/K | N | |
| 28883 | G/C | missense | 204 | G/R | N |
NA: Not applicable.
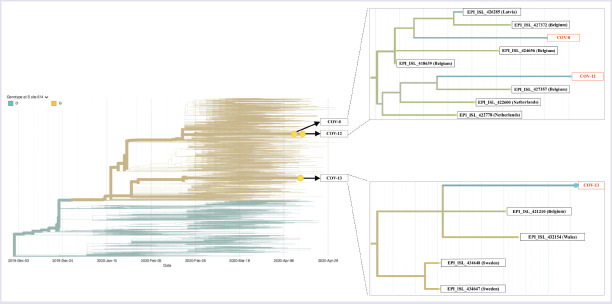
Phylogenetic analysis of the three isolates in this study showed that COV-8 and COV-12 were closely clustered with isolates from Belgium, Netherlands, and Latvia, whereas COV-13 was clustered with Sweden, Belgium and Wales isolates (Figure 3).
FIGURE 2.
Inferred phylogeny and isolates in this study with their closely clustered sequences.
Retrieved and adapted from www.nextstrain.org
DISCUSSION
SARS-CoV-2 is a novel coronavirus that infected more than 3 million people leading to approximately 250,000 deaths globally as of May 2020 [1]. As of May 2020, more than 3,500 patients died in Turkey due to COVID-19, and most of the reported patients are located in Istanbul. Herein, we report three virus genomes isolated in Istanbul for the first time together with patients’ clinical findings. Patients with various clinical presentations (one asymptomatic, one moderate, and one with severe pulmonary infiltration) were included in this study.
Since the first and most of the current cases in Turkey are located in Istanbul, the characterization of virus samples may help to predict the origins of the initial entry to Istanbul. The Nextstrain website (www.nextstrain.org) provides real-time monitoring of viral isolates around the world, mainly based on publicly accessible GISAID data [25, 26]. As of 1 May 2020, more than ten thousand genomes were uploaded to the GISAID database and nearly five thousand different genomes are available for analysis in Nextstrain online tool. Phylogenetic analysis in Nextstrain online tool showed that three isolates from Istanbul in this study were found to be closely clustered within samples isolated mostly in Belgium (Figure 3). This close relationship with Belgium isolates gives clues of early viral entry, at least in Istanbul, may have originated from travelers from European countries.
GISAID classified three large clades, namely S, G and V. Clades were named based on variants L84S in ORF8 (S clade), D614G in S gene (G clade), and G251V in ORF3a (V clade). Three isolates in this analysis carried the D614G variant in the S gene, indicating they are all in G clade, which was mostly detected in European countries. An increased number of patients should be analyzed to enlighten the effects of viral genetic changes on clinical outcomes.
To conclude, we analyzed three SARS-CoV-2 positive individuals in Istanbul, where the epicenter of the pandemic in Turkey. All three viral isolates carried the D614G marker variant indicating the isolates belong to clade G, which encompasses mostly European countries according to GISAID classification. All three virus samples were located in clusters, including isolates from Belgium. As virus surveillance studies are ongoing worldwide, country-wide efforts would also support understanding the local spread of the disease but also evaluate the effectiveness of precautions, such as travel restrictions on disease spread in the country.
Data Availability
Viral genome sequences in this research were deposited in the Global Initiative on Sharing All Influenza Data (GISAID; www.gisaid.org), with accession numbers EPI_ISL_427391, EPI_ISL_428346, and EPI_ISL_428368.
Acknowledgements
We acknowledge the authors, originating and submitting laboratories of the sequence data shared through GISAID’s EpiCOV Database.
Footnotes
Informed Consent: Written informed consent was obtained from all participating patients.
Ethics Committee Approval: This study was approved by the ethics committee of the Umraniye Training and Research Hospital (B.10.1.TKH.4.34.H.GP.0.01/95).
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: This work was supported by Health Institutes of Turkey (TUSEB) (Grant No: 2020TK02-8686/8799)
Authorship Contributions: Concept – IK, LD, GDD; Design – IK, TKA, LD, GDD; Supervision – LD, GDD; Fundings – LD, GDD; Materials – TKA, NBA, AI, GA, JY, BK, ASO, LNA, YKD, MA, OAD, LD, GDD; Data collection and/or processing – IK, TKA, NBA, AI, GA, JY, BK, ASO, LNA, NDC, YKD, MA, OAD, LD, GDD; Analysis and/or interpretation – IK, TKA, NBA, AI, GA, JY, BK, ASO, LNA, NDC, YKD, MA, OAD, LD, GDD; Literature review – IK, NDC, TKA, GDD; Writing – IK, TKA, NDC, NBA, OAD, LD, GDD; Critical review – IK, TK, NBA, OAD, LD, GDD.
REFERENCES
- 1.World Health Organization. Coronavirus disease 2019 (COVID-19):situation report, 107. [Accessed May 15, 2020]. Available at: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200506covid-19-sitrep-107.pdf?sfvrsn=159c3dc_2 .
- 2.Coronaviridae Study Group of the International Committee on Taxonomy of Viruses. The species Severe acute respiratory syndrome-related coronavirus:classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5:536–44. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dallavilla T, Bertelli M, Morresi A, Bushati V, Stuppia L, Beccari T, et al. Bioinformatic analysis indicates that SARS-CoV-2 is unrelated to known artificial coronaviruses. Eur Rev Med Pharmacol Sci. 2020;24:4558–64. doi: 10.26355/eurrev_202004_21041. [DOI] [PubMed] [Google Scholar]
- 4.Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–3. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perlman S, Netland J. Coronaviruses post-SARS:update on replication and pathogenesis. Nat Rev Microbiol. 2009;7:439–50. doi: 10.1038/nrmicro2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zumla A, Hui DS, Perlman S. Middle East respiratory syndrome. Lancet. 2015;386:995–1007. doi: 10.1016/S0140-6736(15)60454-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jin X, Lian JS, Hu JH, Gao J, Zheng L, Zhang YM, et al. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut. 2020;69:1002–9. doi: 10.1136/gutjnl-2020-320926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eliezer M, Hautefort C, Hamel AL, Verillaud B, Herman P, Houdart E, et al. Sudden and Complete Olfactory Loss Function as a Possible Symptom of COVID-19. JAMA Otolaryngol Head Neck Surg 2020 Apr 8 [Epub ahead of print] doi: 10.1001/jamaoto.2020.0832. doi:10.1001/jamaoto.2020.0832. [DOI] [PubMed] [Google Scholar]
- 9.The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team. The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) - China, 2020. China CDC Weekly. 2020;2:113–22. [PMC free article] [PubMed] [Google Scholar]
- 10.Nishiura H, Kobayashi T, Miyama T, Suzuki A, Jung SM, Hayashi K, et al. Estimation of the asymptomatic ratio of novel coronavirus infections (COVID-19) Int J Infect Dis. 2020;94:154–5. doi: 10.1016/j.ijid.2020.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mizumoto K, Kagaya K, Zarebski A, Chowell G. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. Euro Surveill. 2020;25:2000180. doi: 10.2807/1560-7917.ES.2020.25.10.2000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garg S, Kim L, Whitaker M, O'Halloran A, Cummings C, Holstein R, et al. Hospitalization Rates and Characteristics of Patients Hospitalized with Laboratory-Confirmed Coronavirus Disease 2019 - COVID-NET, 14 States, March 1-30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:458–64. doi: 10.15585/mmwr.mm6915e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China:a retrospective cohort study. Lancet. 2020;395:1054–62. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Belouzard S, Chu VC, Whittaker GR. Activation of the SARS coronavirus spike protein via sequential proteolytic cleavage at two distinct sites. Proc Natl Acad Sci U S A. 2009;106:5871–6. doi: 10.1073/pnas.0809524106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ou X, Liu Y, Lei X, Li P, Mi D, Ren L, et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat Commun. 2020;11:1620. doi: 10.1038/s41467-020-15562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh CL, Abiona O, et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–3. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berngruber TW, Froissart R, Choisy M, Gandon S. Evolution of virulence in emerging epidemics. PLoS Pathog. 2013;9:e1003209. doi: 10.1371/journal.ppat.1003209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhan SH, Deverman BE, Chan YA. SARS-CoV-2 is well adapted for humans. What does this mean for re-emergence? bioRxiv. 2020:1–28. [Google Scholar]
- 19.Doganay L, Ozdil K, Memisoglu K, Katrinli S, Karakoc E, Nikerel E, et al. Integrating personalized genomics into Turkish healthcare system:A cancer-oriented pilot activity of Istanbul Northern Anatolian Public Hospitals with GLAB. North Clin Istanb. 2017;4:1–3. doi: 10.14744/nci.2017.38980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andrews S. FastQC:a quality control tool for high throughput sequence data. [Accessed May 15, 2020]. Available at: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/
- 21.Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet Journal. 2011;17:10–2. [Google Scholar]
- 22.Li H. Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. arXiv. 2013:1–3. [Google Scholar]
- 23.Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, et al. 1000 Genome Project Data Processing Subgroup. The Sequence Alignment/Map format and SAMtools. Bioinformatics. 2009;25:2078–9. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grubaugh ND, Gangavarapu K, Quick J, Matteson NL, De Jesus JG, Main BJ, et al. An amplicon-based sequencing framework for accurately measuring intrahost virus diversity using PrimalSeq and iVar. Genome Biol. 2019;20:8. doi: 10.1186/s13059-018-1618-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hadfield J, Megill C, Bell SM, Huddleston J, Potter B, Callender C, et al. Nextstrain:real-time tracking of pathogen evolution. Bioinformatics. 2018;34:4121–3. doi: 10.1093/bioinformatics/bty407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sagulenko P, Puller V, Neher RA. TreeTime:Maximum-likelihood phylodynamic analysis. Virus Evolution. 2018;4:vex042. doi: 10.1093/ve/vex042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.T.C. Sağlık Bakanlığı. COVID-19 (SARS-CoV-2 enfeksiyonu) rehberi. [Accessed May 15, 2020]. Available at: https://covid19bilgi.saglik.gov.tr/depo/rehberler/COVID-19_Rehberi.pdf?type=file .
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Viral genome sequences in this research were deposited in the Global Initiative on Sharing All Influenza Data (GISAID; www.gisaid.org), with accession numbers EPI_ISL_427391, EPI_ISL_428346, and EPI_ISL_428368.