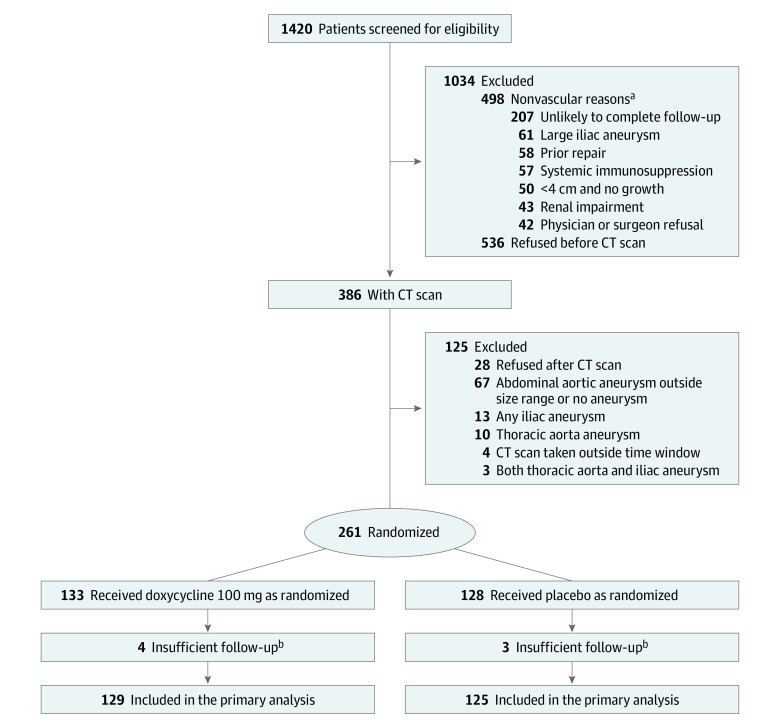
Figure 1. Recruitment, Randomization, and Patient Flow Through the Non-Invasive Treatment of Abdominal Aortic Aneurysm Clinical Trial.
aPatients may have had more than 1 reason for exclusion, less frequently were allergy or intolerance of tetracycline (n = 12), use of tetracycline in last 6 months (n = 19), long-term infection and antibiotic use (n = 20), enrollment in another clinical trial (n = 15), known genetic syndrome (n = 2).
bPatients did not take treatment or did not return for follow-up.
CT indicates computed tomography.