Table 1.
Histone deacetylase inhibitors in clinical trial
| Class | Compound | Structure | Isotype selectivitya,b | Company | Stage |
|---|---|---|---|---|---|
| Hydroxamic acid | Vorinostat/Zolinza (SAHA) | Class I, IIa, IIb, IV | Merck | USFDA approval for CTCL | |
| Belinostat/PXD101 | Class I, IIa, IIb, IV | TopoTarget | Phase II | ||
| Panobinostat/LBH589 | 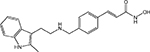 |
Class I, IIa, IIb, IV | Novartis | Phase III | |
| CRA-024781 (PCI 24781) | 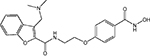 |
Class I, IIb | Celera Genomics | Phase I | |
| ITF2357 | Class I, II Class I, II | Italfarmaco | Phase II | ||
| SB939 | 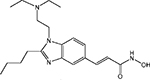 |
ND | S*BIO | Phase I | |
| R306465 (JNJ-16241199) | 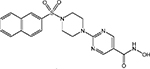 |
Class I | Johnson & Johnson | Phase I | |
| Cyclic peptide | Romidepsin/depsi peptide (FK 228) | 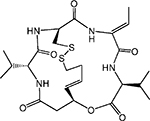 |
HDAC 1, 2, 4, 6 | Gloucester | Phase II |
| Aliphatic acid | VPA | 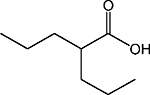 |
Class I, IIa | G2M | Phase II |
| Benzamide | SNDX-275/entinostat (MS-275) | 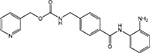 |
HDAC 1, 2, 3, 9 | Syndax/Bayer Schering Pharma AG | Phase II |
| MGCD0103 | 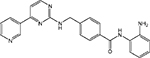 |
HDAC 1, 2, 3, 11 | MethylGene | Phase II |
CTCL, cutaneous T-cell lymphoma; HDAC, histone deacetylase; ND, isotype selectivity not known; USFDA, US Food and Drug Administration; VPA, valproic acid.
Class I: HDAC 1, 2, 3, 8; class IIa: HDAC 4, 5, 7, 9; class IIb: HDAC 6, 10; class IV: HDAC 11.
