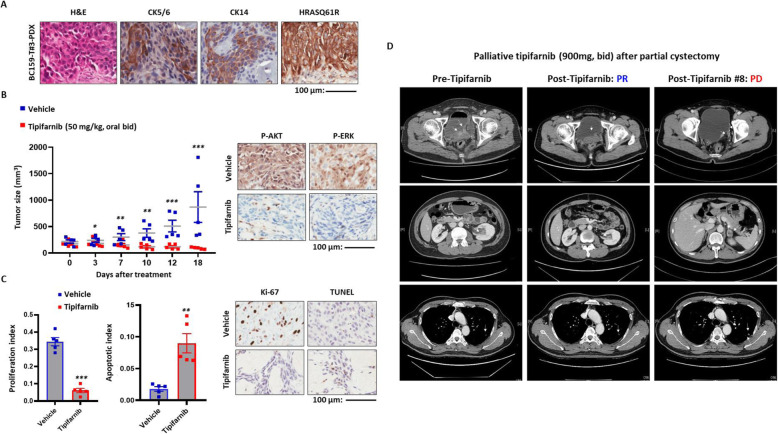
Fig. 2.
In vivo and clinical efficacy of the HRAS-targeting treatment. a Immunohistochemistry staining of protein markers of basal squamous subtype (CD5/6, CD14) and mutant HRASQ61R protein performed on tumor sections from patient-derived xenograft (PDX) of BC159-T#3. Abbreviation: H&E, hematoxylin and eosin. Scale bar, 100 μm. b Tumor sizes were measured in BC159-T#3 PDX administered tipifarnib (50 mg/kg) or vehicle as control. ***P < 0.001, **P < 0.01, *P < 0.05, Error bars indicate standard deviation (SD) (left panel). Immunohistochemistry staining of phosphorylated-AKT (P-AKT) and ERK (P-ERK) using PDX to evaluate the inhibitory effects of tipifarnib on HRAS downstream pathways (right panel). c Comparison of tumor cell proliferation and apoptosis between the tipifarnib and vehicle groups. ***P < 0.001, **P < 0.01, *P < 0.05. Error bars indicate SD (left panel). Immunohistochemistry staining of protein markers of proliferation (Ki-67) and apoptosis (TUNEL) performed on PDX tumor sections to validate the therapeutic efficacy (right panel). d Clinical responses to tipifarnib of the patient evaluated by serial chest–abdomen–pelvis computed tomography (CT). Left panel, before initiation of tipifarnib; middle panel, partial response to tipifarnib; right, the progression of primary tumor and lung metastases due to the resistance to tipifarnib