FIGURE 1.
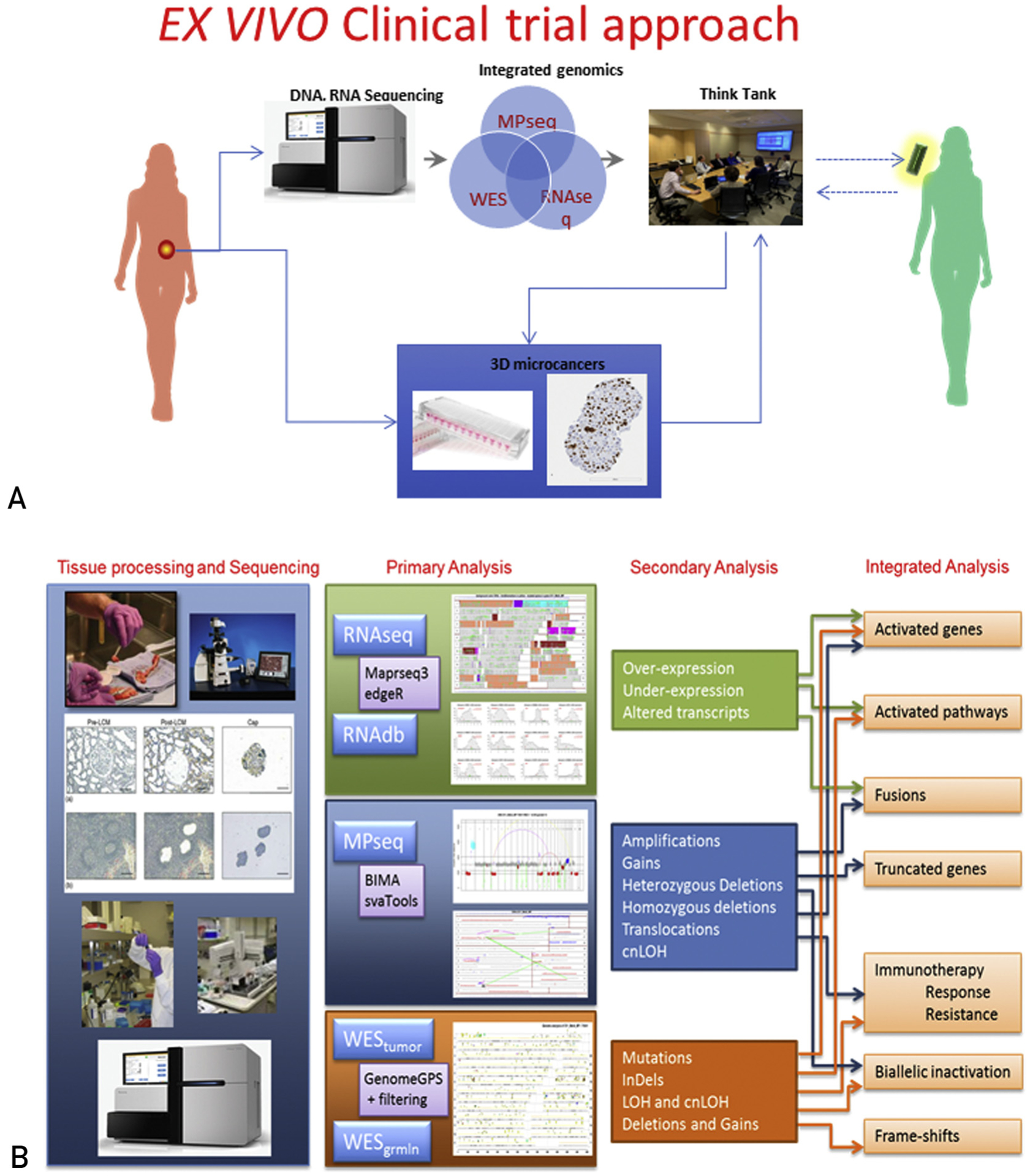
(A) Protocol schema. Tumor tissue of sufficient cellularity is split in two, and is either (1) flash frozen for subsequent pathology review, isolation of genomic material, and DNA/RNA sequencing, or (2) suspended in tissue culture media, minced, and cryopreserved for subsequent 3-dimensional microcancer analysis. Integrated genomic data are reviewed by a molecular tumor board to inform agent selection for screening in the 3-dimensional models. The molecular tumor board reconvenes to review drug sensitivity data in combination with the clinical and genomic data to generate an informed list of treatment regimens. Findings of potential clinical relevance are verified in a CAP/CLIA certified clinical laboratory as appropriate and used to direct therapy at the discretion of the health care provider. (B) Overall schematic illustrating the flow of the integrated Genomics pipeline. CAP = College of American Pathologists; CLIA = Clinical Laboratory Improvement Amendments; cnLOH = copy neutral loss of heterozygosity; MPseq = mate-pair sequencing; RNAseq = RNA sequencing; WES = whole-exome sequencing.
