Abstract
Diabetes emerged as major risk factor for severe acute respiratory syndrome (SARS) and adverse outcome in patients with the coronavirus disease 2019 (COVID-19). Nevertheless, the role of admission hyperglycemia in patients with COVID-19 has not been well-explored, yet. With this retrospective analysis, we report for the first time that hyperglycemia on day-1 is the best predictor of radiographic imaging of SARS-CoV2, regardless of the past medical history of diabetes. Admission hyperglycemia should not be overlooked, but adequately treated to improve the outcomes of COVID-19 patients with our without diabetes.
Keywords: COVID-19, Diabetes, Hyperglycemia, SARS-Cov2
1. Introduction
Diabetes emerged as major risk factor for severe acute respiratory syndrome (SARS) and adverse outcome in patients with the coronavirus disease 2019 (COVID-19) [1], [2], [3], [4], [5]. Diabetes is characterized by chronic hyperglycemia affecting the immune response to the coronavirus. However, acute or stress hyperglycemia may lead to further complications in COVID-19 patients, irrespectively of a past history of diabetes. Stress-induced hyperglycemia is a well-described body’s response and maladaptive mechanism. An early report from Wuhan, China, described hyperglycemia in 51% of the patients with COVID-19. Nevertheless, the role of admission hyperglycemia in patients with COVID-19 has not been well-explored, yet. In this report we sought to evaluate the correlation of admission glucose levels with clinical and imaging respiratory parameters in COVID-19 patients with our or without diabetes.
1.1. Population and data collection
We retrospectively collected data from 85 patients with laboratory-confirmed COVID-19 infection who were admitted at UHealth Tower (UHT), University of Miami Hospital between March 4 and April 4, 2020. A confirmed case of COVID-19 was defined by a positive result on a reverse-transcriptase–polymerase-chain-reaction (RT-PCR) assay of a specimen collected on a nasopharyngeal swab. Clinical specimens for COVID-19 were obtained in accordance with Centers for Disease Control and Prevention (CDC) guidelines [6]. We included only laboratory-confirmed cases.
Deidentified data from UHT electronic medical records were collected. We obtained demographic data, information on clinical symptoms or signs at presentation, and laboratory and radiologic results during 5 days of hospital admission. All laboratory tests and chest radiography of the chest, were performed at as per hospital COVID-19 protocol.
CXRs and standard laboratory tests, including multiple blood glucose levels, plasma interleukin-6 (IL-6), c reactive protein (CRP), ferritin and D-dimer were collected from day-1 to day-5 from each patient.
Acute respiratory distress syndrome (ARDS) was defined as acute-onset hypoxemia with bilateral pulmonary opacities on chest imaging that were not fully explained by congestive heart failure or other forms of volume overload [2]. Chest radiograms (CXR) changes were scored as followed: 0 = clear, 1 = focal opacity, 2 = multifocal opacity; 3 = bilateral pulmonary opacities suggestive of ARDS.
CXRs were obtained in the antero-posterior orientation using one of two portable chest x-ray machines, the Mobilett Mira Max by Siemens Healthineers or the AccE GM85 by Samsung Electronics. The detector plate was placed behind the back of the patient while supine, with the beam penetrating from anterior to posterior. Arms were kept at the sides of the chest and the patient was asked to do a suspended inspiration, whenever possible
1.2. Statistical analysis
Continuous variables are presented as means with their standard deviations (SDs) or medians for skewed data. Relations between study variables were calculated using bivariate regression analysis with Pearson or Spearman (rho) coefficient for skewed data with two-tailed p < 0.05 indicating statistical significance. Statistical analysis was performed using SPSS 26, Armonk, NY: IBM Corp.
2. Results
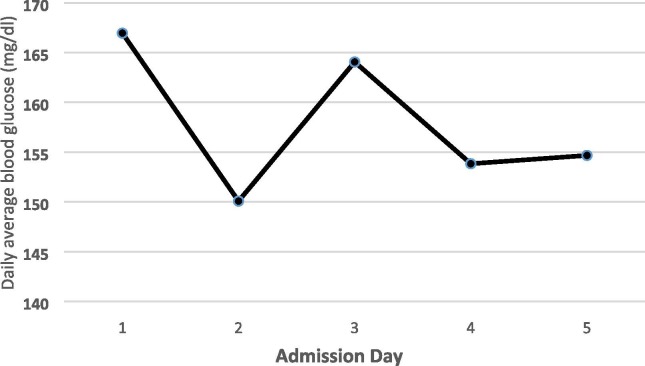
In summary, patients’ age ranged from 31 to 95 years old with an average of 65 years old, with more men than women (49 vs 36). Twenty-seven out of 85 patients had past medical history for diabetes (32%), 16 patients were on oral anti-diabetic agents, including metformin, dipeptidyl peptidase 4 (DPP4)-inhibitors and sulfonylureas; 7 on a combination of insulin and orals and 3 were on only insulin therapy. Average blood glucose levels on day-1 was 166 ± 81 mg/dl with range between 65 and 423 mg/dl. HemoglobinA1c was also collected and found to range from 5.7 to 15.2% with a median of 7%. Patients were started on subcutaneous fast acting (lispro) and long acting (glargine) insulin, as per hospital protocol. Daily average blood glucose levels improved accordingly, Fig. 1 . Total daily insulin units was small ranging from 5 to 15 units daily. Five patients died during the 5 days of admission.
Fig. 1.
Glucose changes during the admission in COVID-19 patients with or without diabetes.
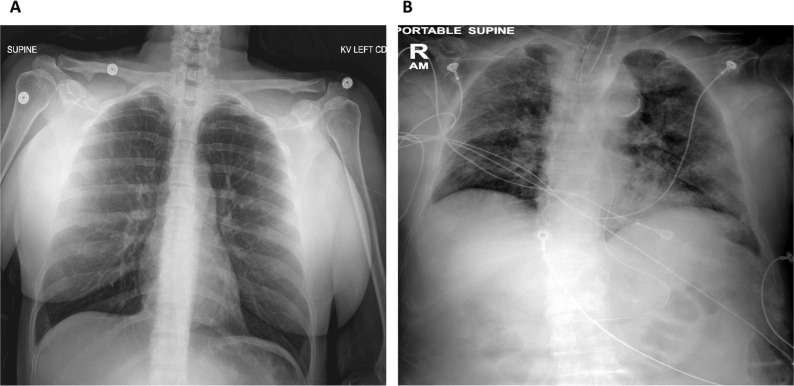
In a simple regression analysis daily average blood glucose was positively correlated with daily CXR findings of ARSD (r = 0.46, p = 0.03 for day-1; r = 0.46, p = 0.05 for day 2 and r = 0.75, p = 0.03 for day 4, respectively). BMI was significantly correlated with day-1 CXR. The correlation between age and chest radiography was not quite statistically significant (r = 0.2, p = 0.09). None of the other parameters correlated with chest radiography. Hence, blood glucose, BMI and age were entered in a multiple regression model to identify the best independent correlate of chest radiographic score, our dependent variable. Day-1 average blood glucose was the strongest independent variable predicting SARS-CoV2 radiographic imaging. Glucose remained the best predictor, also when body temperature was entered in the model (Table 1 ). Interestingly, 4 out of 5 patients who were taking DPP4i prior to the admission had normal CXR on admission day-1. Fig. 2 shows the striking CXR differences between a sample patient with normal glucose and one with hyperglycemia on admission day-1. None of these patients had history of diabetes.
Table 1.
Predictors of SARS-CoV2 chest radiography in patients with or without diabetes.
| Model 1 | B | Std. Error | Beta | t | Sig. |
|---|---|---|---|---|---|
| Age | −0.017 | 0.016 | −0.255 | −1.083 | 0.307 |
| BMI | −0.012 | 0.034 | −0.083 | −0.354 | 0.732 |
| Glucose | 0.015 | 0.004 | 0.800 | 3.754 | 0.005 |
| Model 2 | B | Std. Error | Beta | t | Sig. |
| Age | −0.017 | 0.012 | −0.253 | −1.443 | 0.187 |
| BMI | −0.013 | 0.025 | −0.091 | −0.522 | 0.616 |
| Glucose | 0.017 | 0.003 | 0.912 | 5.595 | 0.001 |
| Temperature | 0.425 | 0.147 | 0.457 | 2.884 | 0.020 |
Multiple regression analysis models with chest radiographic score as dependent variable and age, body mass index (BMI), average daily glucose levels at day 1 (Glucose) and body temperature at day 1 as independent variables; p < 0.05 was considered statistically significant (Sig).
Fig. 2.
(A) anterior-posterior chest x-ray imaging of a female COVID-19 patient with normal blood glucose levels on admission day-1 and no radiographic pathologic findings. Patient was discharged home; (B) anterior-posterior chest x-ray imaging of a male COVID-19 patient with hyperglycemia on admission day-1 who developed SARS. Patient died.
3. Discussion
This reports highlights for the first time that admission hyperglycemia is the best predictor of radiographic imaging of SARS-CoV2, regardless of the past medical history of diabetes. Acute hyperglycemia may lead to an abnormal inflammatory and immune response contributing to the development and progression of the radiographic findings of ARDS in patients with COVID-19. No conclusion on the role of diabetes treatment, such as DPP-4 inhibitors or ACE inhibitors recently suggested as possible targeting treatment [7], [8], could be drawn from this report. However, the finding that most of the diabetic patients who were on home regimen with DPP4 inhibitors had no radiological findings of ARSD deserves further investigations.
Admission hyperglycemia should not be overlooked, but adequately treated to improve the outcomes of COVID-19 patients with our without diabetes.
Funding
The authors received no funding from an external source.
Declaration of Competing Interest
The authors declare no conflict of interest.
References
- 1.Gentile S., Strollo F., Ceriello A. COVID-19 infection in Italian people with diabetes: Lessons learned for our future (an experience to be used) Diabetes Res Clin Pract. 2020;4(162):108137. doi: 10.1016/j.diabres.2020.108137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fang L., Karakiulakis G., Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;8(4):e21. doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet; 2020. pii: S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed]
- 4.Remuzzi A, Remuzzi, G. COVID-19 and Italy: what next? Lancet; 2020 https://doi.org/10.1016/S0140-6736(20)30627-9.
- 5.Bhatraju P.K., Ghassemieh B.J., Nichols M., Kim R., Jerome K.R., Nalla A.K. Covid-19 in critically ill patients in the seattle region - case series. N Engl J Med. 2020;30 doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.https://www.cdc.gov/coronavirus/2019-ncov/lab/guidelines-clinical-specimens.html.
- 7.Iacobellis G. COVID-19 and Diabetes: can DPP4 inhibition play a role? Diabetes Res Clin Pract. 2020:108125. doi: 10.1016/j.diabres.2020.108125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pal R., Bhadada S.K. Should anti-diabetic medications be reconsidered amid COVID-19 pandemic? Diabetes Res Clin Pract. 2020;163 doi: 10.1016/j.diabres.2020.108146. [DOI] [PMC free article] [PubMed] [Google Scholar]