Figure 3.
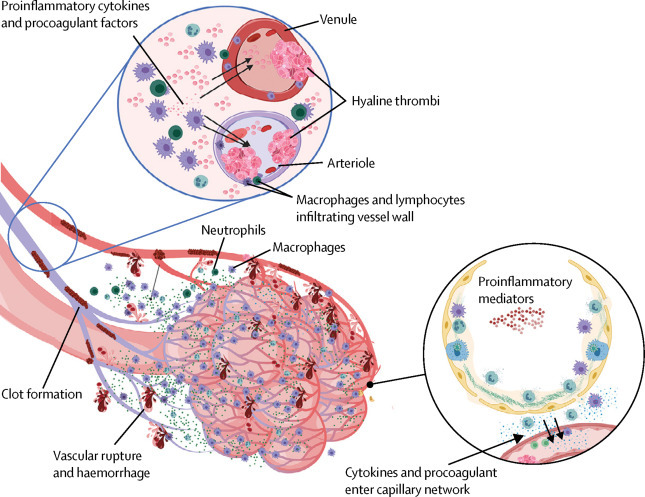
Pulmonary intravascular coagulopathy in COVID-19 pneumonia
Scheme showing how extensive COVID-19 lung involvement with large anatomical interface between infected type II pneumocytes, extensive interstitial immunocyte activation similar to macrophage activation syndrome, and the extensive pulmonary microvascular network, triggers diffuse pulmonary bed extrinsic inflammation with immunothrombosis. This inflammation causes microthrombotic immunopathology that leads to right ventricular stress and contributes to mortality. Diffuse type II pneumocyte centric pathology with extension into the interstitium leads to extensive pulmonary macrophage recruitment and activation, resulting in a clinical picture similar to local macrophage activation syndrome. Proinflammatory and procoagulants gain access to the capillary network (lower circle). The low pressure nature of the vascular system and thin vessel walls in and proximal to the alveolar network triggers immunothrombosis by various mechanisms (eg, local elevations in proinflammatory cytokines), vessel wall tissue damage with tissue factor production, and direct injury to small vessels. Vigorous fibrinolytic activity (detected early by D-dimer elevation) might not keep in check the extensive microthrombi formation, leading to the evolution of pulmonary infarction, haemorrhaging, and pulmonary hypertension induced by pulmonary intravascular coagulopathy, all of which are driven by COVID-19 inflammation. Thus, risk factors for cardiovascular disease might increase the likelihood of death in severe COVID-19 inflammation. COVID-19=coronavirus disease 2019.
