Highlights
-
•
Immunization for older adults is an important strategy for healthy aging.
-
•
We conducted a country archetype analysis of vaccine decisions and implementation.
-
•
We found four distinct archetypes amongst the countries analyzed.
-
•
Understanding country drivers & facilitators could help inform global strategies.
Keywords: Adult immunization, Decision-making, Implementation, Vaccine, Policy, Archetype, Healthy aging, Older adults, Life-course, COVID-19
Abstract
The global population of adults over 65 years of age is growing rapidly and is expected to double by 2050. Countries will face substantial health, economic and social burden deriving from vaccine-preventable diseases (VPDs) such as influenza, pneumonia and herpes zoster in older adults. It will be essential that countries utilize several public health strategies, including immunization. Understanding the different approaches countries have taken on adult immunization could help provide future learnings and technical support for adult vaccines within life-course immunization strategies. In this study, we describe the priorities and approaches that underlie adult immunization decision-making and implementation processes in 32 high-and-middle-income countries and two territories (“34 countries”) who recommend adult vaccines in their national schedule. We conducted an archetype analysis based on a subset of two dozen indicators abstracted from a larger database. The analysis was based on a mixed-methods study, including results from 120 key informant interviews in six countries and a landscape review of secondary data from 34 countries. We found four distinct archetypes: disease prevention-focused; health security-focused; evolving adult focus; and, child-focused and cost-sensitive. The highest performing countries belonged to the disease prevention-focused and health security archetypes, although there was a range of performance within each archetype. Considering common barriers and facilitators of decision-making and implementation of adult vaccines within a primary archetype could help provide a framework for strategies to support countries with similar needs and approaches. It can also help in developing context-specific policies and guidance, including for countries prioritizing adult immunization programs in light of COVID-19. Further research may be beneficial to further refine archetypes and expand the understanding of what influences success within them. This can help advance policies and action that will improve vaccine access for older adults and build a stronger appreciation of the value of immunization amongst a variety of stakeholders.
1. Background
Older adults are a heterogeneous group in the second half of life [1]. Studies demonstrate that vaccine-preventable diseases (VPDs), including influenza, pneumonia and herpes zoster, account for a substantial portion of premature death and disability in older adults [2], [3]. VPDs also have the potential to cause disability that may lead to additional issues, such as declines in functional ability and quality of life [4]. The economic burden of VPDs is also substantial [3], [5], [6], [7], [8], [9], [10]. The global population of adults over the age of 65 is growing rapidly and is expected to double by 2050 [11]. Although adults are expected to live longer, they are not necessarily living in good health [1]. To be prepared for this demographic change, countries must establish plans and infrastructure that support healthy aging. Aging populations will impact countries’ economies, social security policies, and health systems, as well as affect many aspects of daily living for both the individual and broader society [12]. At a national level, the consequences of an aging population extend beyond the health sector and solutions must be viewed across the life course [1], [12]. It will be essential that countries utilize several strategies to ensure that their older populations age in a healthy manner, including adult immunization [13], [14]. As they have for children, vaccines have the potential to significantly reduce burden of disease and disability, dependence, healthcare costs, and more in older populations [15], [16], [17], [18], [19], [20].
Given the imminence of a growing, worldwide adult population, anticipation of increased health costs has spurred multiple global, regional and national calls for action for policymakers and practitioners to prioritize adult immunization programs and improve uptake [19], [21], [22], [23], [24], [25], [26], [27]. Many high- and middle-income countries have adopted influenza vaccines [28], however few have adopted more than one vaccine for older adults or have comprehensive adult immunization strategies. Countries that have adopted adult vaccines appear to have taken different pathways to policy adoption [29], [30], [31], [32], [33]. Further, to be prepared for healthy aging, countries must think holistically. They will need systems and an appreciation for prioritizing health in older adults to support delivery of vaccines and other crucial interventions [1], [19], [34]. Particularly in the context of COVID-19, preventing further strain on the health system is paramount [53].
The Global Vaccine Action Plan [35] and the Immunization Agenda 2030 [36] call for a life-course approach to immunization, which some countries have begun to implement. Despite similarities in disease burden, demographic profile, and geographic proximity, these countries have taken different approaches and achieved different results in adult vaccine adoption. To understand those differences and explain the factors influencing country decision-making and uptake, we conducted an archetype analysis. This analysis aims to describe the country priorities and approaches that underlie adult immunization decision-making and implementation. By characterizing groups of countries by features other than disease burden, geography or demographics, the analysis seeks to support global efforts to address country needs in strengthening processes for vaccine decision-making and implementation; facilitating sharing of best practices amongst countries with similar characteristics; and providing evidence, system or advocacy support to help countries succeed within their specific context. The archetype analysis does not replace the need for individual country strategies, but groups needs in a way that enables the global community to provide meaningful support across a broader group of countries.
2. Methodology
2.1. Country selection
Thirty-two countries and two territories (herein referred to as 34 countries) were selected for analysis. Countries selected had high proportions of older adults, were geographically diverse, and represented a range of potential archetypes based on their adult vaccine adoption status, financing models, degree of health system centralization, and vaccine coverage. All 34 countries were included in a literature review and data abstraction. Six countries (Argentina, Australia, Canada, Germany, Japan, and the United Kingdom (UK)) were selected for further qualitative research to provide additional depth in a diversity of contexts, approaches and performance. The insights gained in each case country helped characterize archetypes. Each case country, to some degree, prioritizes adult vaccination and recommends and finances one to three adult vaccines, but varies in terms of government and/or healthcare system centralization and adult vaccine coverage rates. The United States (US) was not selected as a case study country, due to IVAC’s working knowledge of the American public immunization system and the abundance of publicly available peer-reviewed articles and government documentation on older adult immunization.
2.2. Ethics review
The study plan was reviewed by the Johns Hopkins Bloomberg School of Public Health Institutional Review Board and deemed to be non-human subjects research.
2.3. Indicator selection
Domains (Table 1 ) were identified as part of a framework of potential barriers and facilitators for adult vaccine decision-making: country characteristics, adult vaccine/aging policies and decision-making, health immunization systems, uptake, and stakeholders and champions. These domains were the subject of key informant interviews conducted in six case countries and a concurrent landscape analysis of the 34 countries. A series of indicators were subsequently identified, informed by previous research [37], an adult vaccine situational analysis [38], and the case study interviews.
Table 1.
Research domains and illustrative indicators.
| Domain | Illustrative Indicators | Number of Indicators in Domain |
|---|---|---|
| Country Characteristics | Life expectancies Percent of population aged 65 and older Income group Disease burden |
32 |
| Health & Immunization Systems | Insurance systems Registries Financing mechanisms Procurement Providers Barriers to access |
>50 |
| Policies & Decision-making | NITAG structures Vaccine-specific recommendations for adults (including target populations, age, by government or other bodies) Immunization laws Country requirements |
28 |
| Adult Vaccine Uptake | Vaccine-specific coverage for adults | 15 |
| Stakeholders & Champions | Key experts & advocates across medical, healthy aging, and other communities | 7 |
A landscape review of the 34 countries was conducted. Research was divided into five domains and data collected on specific indicators, as illustrated above.
2.4. Data collection
A team of abstractors conducted the indicator research. We searched peer-reviewed and grey literature, including government and professional society websites, disease burden and vaccine introduction and program status databases, reports from countries, the World Health Organization (WHO), non-governmental organizations (NGOs), and media articles. We reviewed minutes from national technical advisory groups on immunization (NITAGs) as well as official recommendations and policies for both immunization and aging. Data abstraction was done in English. Where necessary, the process was supplemented by Google translate and student translations for the following languages: Arabic, Chinese, Danish, Dutch, French, German, Japanese, Korean, Portuguese, and Swedish. Quantitative and qualitative data were collected from January 2018 through October 2018 and entered into a Microsoft Excel database. Data were later migrated into Microsoft Access to improve querying capabilities. Sources were recorded for all information and online links provided where possible to enable easy reference. Quality control checks were conducted and aligned with the results obtained from the case studies.
2.5. Case study interviews
Key informant interviews were conducted in 2018 in Argentina, Australia, Canada, Germany, Japan and the UK. Informants included vaccine experts, government and former government officials, healthy aging advocates, economists, and civil society. Respondents were selected using a snowball approach. One-hour face-to-face interviews were conducted by 1–2 people using an interview guide. For each respondent type – technical respondents, economic respondents and health advocacy-focused respondents – unique guides were prepared to ensure the focus of the questions was on their area of expertise. A combination of open-ended questions and probes were used. Topics included respondent background; country health priorities; key players and stakeholders; robustness of the process; drivers of decisions and uptake. We used scales of very important, moderately important, not important, and don’t know/not sure to ascertain respondents’ perceptions about drivers or degree of agreement with certain statements and to map responses. Interviews were transcribed and entered into ATLAS.ti 8 for Mac OS X (a qualitative data analysis software) to conduct a thematic analysis. No identifiers were included to keep the identities of respondents confidential. Descriptive categories of their function (e.g., technical, economic, civil society organization, etc.), were included instead.
2.6. Analysis of performance
Based on the case study findings, we selected indicators that most differentiated countries and created a scoring of 0–2 to rate each country, with 2 meaning that it fit the criterion well and 0 that it did not fit the criterion and/or there was no data available to assess fit (Table 2 ). Each indicator was ascribed a score based upon a qualitative description. Following validation, we found some of our scoring was insufficient in describing qualitative nuance and added an intermediate score of 0.5 to one indicator and 1.5 to four indicators. We scored all countries on 19 indicators related to decision-making (10 indicators) and implementation (9 indicators). Each country received a score and a ranking for both domains (i.e., decision-making and implementation). We recorded the scores in a table, organizing countries by pneumococcal vaccine coverage (high to low). We mapped all countries on a grid using Graph Pad Prism version 7.0.
Table 2.
Scoring.
| Decision-making | |
| Early or late adopter of adult vaccines |
0 = No or late decision-making and adoption of either pneumococcal or herpes zoster vaccine 1 = Follower in decision-making and adoption of at least one vaccine 2 = Leader in decision-making and adoption of one or more vaccines |
| Country-specific policy requirements of manufacturers |
0 = Multiple 1 = One 2 = None |
| Disease burden surveillance |
0 = No surveillance 1 = Some surveillance (mostly of flu and pneumococcal disease) 2 = National surveillance of flu, pneumococcal disease, and herpes zoster |
| NITAG’s prioritization of health security in decision-making |
0 = Small to no priority on health security, or no evidence available 1 = NITAG considers health security, but it is not a main driver of decisions 2 = NITAG considers health security as a main driver of decisions |
| NITAG’s utilization of cost-effectiveness (C-E) data in decision-making |
0 = Small to no focus on C-E, or no evidence available 1 = NITAG considers C-E data, but it is not a main driver 1.5 *= NITAG mostly considers C-E data as a main driver 2 = NITAG considers C-E data as a main driver |
| NITAG has adult vaccine working group(s) |
0 = 0 such working groups 0.5* = NITAG has 0 such working groups, but is involved in other recommending bodies where government is engaged 1 = 1 such working group 2 = Multiple such working groups (as part of a broader vaccine-specific working group or a standalone) |
| Public policy - pneumococcal vaccination for older adults |
0 = PCV or PPSV not recommend, unknown if considered by NITAG 1 = PCV or PPSV considered by NITAG, but not recommended 1.5* = PCV or PPSV was recommend by NITAG, but not yet implemented 2 = PCV recommended by NITAG |
| Public policy - herpes zoster vaccine (HZV) for older adults |
0 = HZV not recommended, unknown if considered by NITAG 1 = HZV considered, but not recommend by NITAG 1.5*= HZV recommended by NITAG, but not yet implemented 2 = HZV recommended by NITAG |
| Publication of Health Aging Strategies |
0 = No healthy aging strategy publicly available 1 = Aging strategy available at the sub-national or national level 1.5*= Sub-national or national aging strategy available that mentions adult immunization, but is over ten years old 2 = National aging strategy available that mentions adult vaccines |
| Publication of National Immunization Strategies |
0 = No immunization strategy publicly available 1 = Only pediatric immunization strategy publicly available 2 = National immunization strategy published and covers both pediatric and adult vaccines |
| Implementation | |
| Vaccine Financing – Level of public financing (for each vaccine) |
0 = Older adults must pay out of pocket 1 = Vaccine covered by private insurance, requires co-pay, or limited coverage is provided in certain geographic areas or at-risk populations 1.5*= Mixed system of payment (covered) 2 = Vaccine is fully funded by the government for all |
| Vaccine Registry (for pediatric and adult populations) |
0= No registry 1= Sub-national or by individual health systems/providers/insurers 2= Centralized |
| Availability of Public Vaccine Coverage Data (for each vaccine) |
0= No evidence of being measured 1= Some public coverage data (at sub-national level and/or by age) 2= Complete public coverage data (at national level and/or across life-course) |
| Advocacy – promotion of adult immunization |
0= No evidence of advocacy for older adult vaccines 1= Few advocacy initiatives 2= Multiple sectors promoting older adult vaccines |
| Influence of individuals or organizational leaders on how older adult immunization program is implemented |
0= No influence 1= Some influence, but data influences more 2= Significant influence of champions or organizational leaders |
| Access – Ease of getting vaccinated as an older adult |
0= Difficult to get vaccinated 1= Somewhat complicated 2= Easy to get vaccinated (multiple locations and/or providers) |
| Equity is a focus in adult vaccine program implementation |
0= No or little evidence of equity focus 1= Some evidence of equity focus 2= Multiple sources of evidence of equity focus |
| Degree of centralization of adult vaccine delivery |
0 = Decentralized 1 = Mixture 2= Centralized |
| Degree of centralization of health system delivery |
0= Decentralized 1= Mixture 2= Centralized |
*0.5 and 1.5 scores added based on feedback during validation process.
Countries were assessed on their adult vaccine implementation and decision-making. Decision-making was scored upon 10 indicators and implementation upon 9 indicators. Each indicator was ascribed a quantitative score, as described above.
2.7. Development of archetypes
We used the case study insights to identify characteristics that could describe the primary driver of a country’s approach to decision-making and implementation (the “archetype”). Based on the qualitative insights, we found four distinct archetypes and placed each country into one archetype. Although countries could fit in more than one archetype, we selected the archetype that most closely fit each country’s primary driver. Thus, country performance does not define each archetype’s description.
2.8. Validation
We transcribed each country’s data into individual country profiles, providing the qualitative description associated with each quantitative score. We asked respondents to indicate their level of agreement (agree/disagree) with each score. We requested the respondents to identify missing data or inaccurate scores, that if corrected, could substantially move a country’s score higher or lower. If there was disagreement, we asked respondents to provide an explanation and publicly available source(s) that support the change they suggested. We reached out to 2–5 experts per country, including a Ministry of Health representative, wherever possible. We provided a slide set describing the project, including objectives, methodology and the archetype map to enable countries to provide meaningful input. Countries that responded are listed (Table 3 ).
Table 3.
Countries responding to validation survey.
| Country | Received survey results | Responded with feedback |
|---|---|---|
| Argentina | ✓ | |
| Australia | ✓ | ✓ |
| Belgium | ✓ | ✓ |
| Brazil | ✓ | ✓ |
| Canada | ✓ | ✓ |
| China | ✓ | ✓ |
| Colombia | ✓ | ✓ |
| Denmark | ✓ | |
| France | ✓ | |
| Germany | ✓ | ✓ |
| Greece | ✓ | |
| Hong Kong | ✓ | ✓ |
| India | ✓ | ✓ |
| Ireland | ✓ | |
| Italy | ✓ | ✓ |
| Japan | ✓ | ✓ |
| South Korea | ✓ | |
| Malaysia | ✓ | ✓ |
| Mexico | ✓ | ✓ |
| Netherlands | ✓ | ✓ |
| New Zealand | ✓ | ✓ |
| Norway | ✓ | |
| Peru | ✓ | |
| Philippines | ✓ | ✓ |
| Russia | ✓ | |
| Saudi Arabia | ✓ | ✓ |
| Spain | ✓ | |
| Sweden | ✓ | ✓ |
| Switzerland | ✓ | ✓ |
| Taiwan | ✓ | |
| Turkey | ✓ | |
| UAE | ✓ | |
| UK | ✓ | ✓ |
| USA | ✓ | ✓ |
The results of the archetype analysis was shared with experts representing all 34 countries. 21 countries responded and participated in the validation process.
3. Results
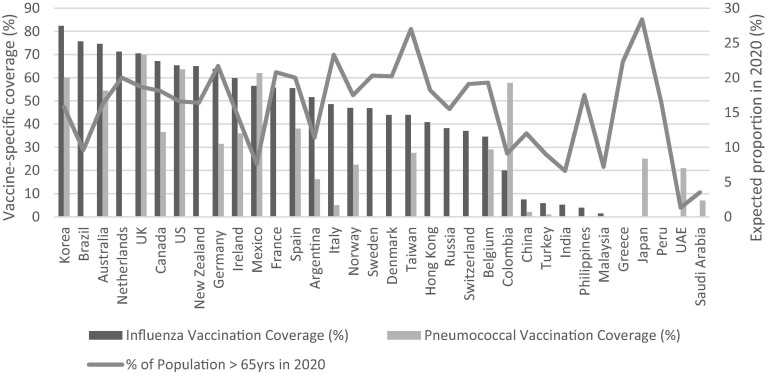
Thirty of the 34 countries analyzed had more than 20% of their total population adults over 50 years of age. Twenty-four countries had over 10% of their population over 65 years of age. High-income countries and upper middle-income countries, had older populations than lower-middle and low-income countries (Table 4 ), but the age of populations did not predict the number of vaccines adopted for their adult populations nor uptake of influenza or pneumococcal vaccine (Fig. 1 ).
Table 4.
Proportion of older adults as a percentage of the total population: 2020 and 2050.
| 2020 | 2050 | 2020 | 2050 | |
|---|---|---|---|---|
| % Population 50+ | % Population 50+ | % Population 65+ | % Population 65+ | |
| World | 24.2 | 32.7 | 9.3 | 15.9 |
| High-income countries | 37.7 | 45.2 | 18.4 | 26.9 |
| Upper-middle-income countries | 29.1 | 42.3 | 10.8 | 22.5 |
| Lower-middle-income countries | 17.8 | 28.0 | 5.9 | 11.7 |
| Low-income countries | 10.5 | 16.2 | 3.3 | 5.4 |
| Country | ||||
| Argentina | 25.3 | 34.9 | 11.4 | 17.3 |
| Australia | 34.0 | 40.2 | 16.2 | 22.8 |
| Belgium | 39.4 | 44.8 | 19.3 | 26.9 |
| Brazil | 25.5 | 43.3 | 9.6 | 22.7 |
| Canada | 38.6 | 44.4 | 18.1 | 25.0 |
| China | 32.8 | 47.2 | 12.0 | 26.1 |
| Colombia | 24.1 | 41.6 | 9.1 | 21.0 |
| Denmark | 40.1 | 42.9 | 20.2 | 24.2 |
| France | 40.1 | 45.1 | 20.8 | 27.8 |
| Germany | 44.7 | 49.0 | 21.7 | 30.0 |
| Greece | 43.2 | 54.0 | 22.3 | 36.2 |
| Hong Kong | 42.2 | 53.8 | 18.2 | 34.7 |
| India | 19.4 | 32.8 | 6.6 | 13.8 |
| Ireland | 31.8 | 42.9 | 14.6 | 26.6 |
| Italy | 45.7 | 54.2 | 23.3 | 36.0 |
| Japan | 47.4 | 55.3 | 28.4 | 37.7 |
| Korea | 39.7 | 59.0 | 15.8 | 38.1 |
| Malaysia | 20.7 | 37.5 | 7.2 | 17.0 |
| Mexico | 21.1 | 35.5 | 7.6 | 17.0 |
| Netherlands | 41.3 | 46.7 | 20 | 28 |
| New Zealand | 35.2 | 42.4 | 16.4 | 23.9 |
| Norway | 36.4 | 42.6 | 17.5 | 24.0 |
| Peru | 22.6 | 37.2 | 8.7 | 18.9 |
| Philippines | 17.2 | 28.5 | 5.5 | 11.8 |
| Russia | 35.4 | 41.0 | 15.5 | 22.9 |
| Saudi Arabia | 15.1 | 36.6 | 3.5 | 17.2 |
| Spain | 41.3 | 53.5 | 20.0 | 36.8 |
| Sweden | 38.8 | 42.9 | 20.3 | 14.6 |
| Switzerland | 40.5 | 47.2 | 19.1 | 28.6 |
| Taiwan | 38.3 | 55.7 | 15.8 | 35.0 |
| Turkey | 23.6 | 39.3 | 9.0 | 20.9 |
| UAE | 11.2 | 26.4 | 1.3 | 16.1 |
| UK | 37.9 | 43.8 | 18.7 | 25.3 |
| USA | 35.6 | 40.8 | 16.6 | 22.4 |
Data Source: https://population.un.org/wpp/DataQuery/.
Expected older adult populations are shown by income status groups and by country for 2020 and 2050. Estimates are presented for adults over 50 years of age (50+) and over 65 years of age (65+).
Fig. 1.
Current uptake of adult vaccines compared to expected proportion of older population in 2020, by country. Sources: Vaccine coverage based on multiple sources in IVAC adult vaccine database (2018). Proportion of older adult population from UN Population Trends (2019). Countries are plotted on the x axis, with national influenza (in blue) and pneumococcal vaccine coverage (in orange) plotted on the y-axis. Also plotted (in grey) on the y-axis is an estimate of the older adult population in 2020. Vaccine coverages and expected older adult population vary within and among countries. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.1. Case studies
One hundred and twenty key informant interviews were conducted in the six case countries, including a range of respondents covering health, immunization, government policy, aging and economics. Overall, respondents in Australia and the UK had the greatest degree of confidence in their country’s ability to make decisions about new vaccines for adults and implement programs. The UK respondents attributed their country’s ability to make decisions on the broadest variety of factors and only the influence of advocacy and bringing a variety of perspectives into decision-making was ranked lower. Respondents in Canada expressed a high degree of confidence in their country’s ability to make decisions but reported lower confidence in their government’s level of priority for adult vaccines, access to providers and financing. Germany had similar perceptions to Canada in that the decision-making process was strong, but respondents questioned the ability to implement, ease of access and the variety of advocacy efforts. Argentine and Japanese respondents had lower composite ratings on their perception of their country’s capability to make decisions and implement adult immunization than the other countries but rated their country’s commitment to adult health and the variety of perspectives highly. Argentine respondents also rated their NITAGs as capable. Both Argentine and Japanese respondents had less confidence in ease of access, financing, surveillance for adults.
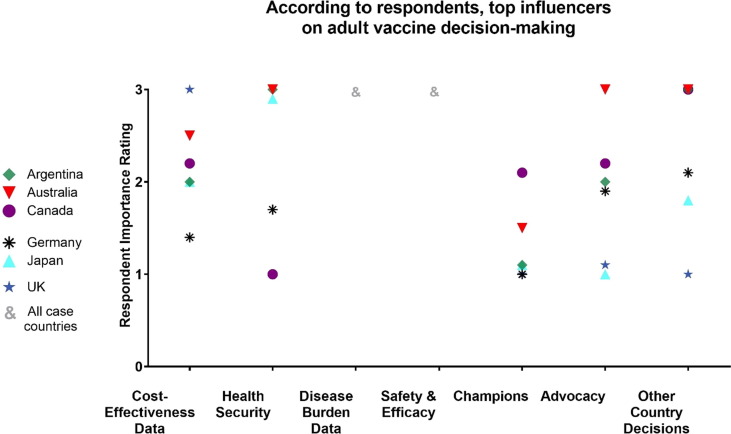
We also noted varying approaches to how cost-effectiveness (C-E) data were used both amongst case study countries and other countries that the respondents described. A few countries, including the UK, used C-E thresholds in their adoption decisions. Other countries considered C-E data but did not use it. In some countries, childhood vaccination was viewed as a higher priority, particularly for funding. Some respondents in Germany even went as far as to state that putting adult health before child health would be unethical. In Japan, we saw no evidence of C-E studies used in their NITAG decisions, although safety studies were a requirement (Fig. 2 ).
Fig. 2.
Drivers of Decision-Making and Implementation. Respondent ratings of how important 8 factors are to country decision-making on adult vaccines. The coding scale used: (0) Unsure or no comment, (1) no influence, (2) little influence, (3) strong influence.
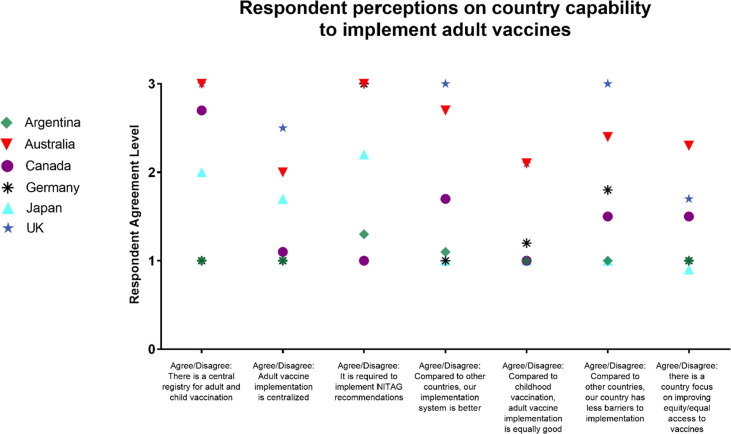
Similar to decision-making, factors influencing implementation varied by country (Fig. 3 ). In the case countries, we saw recommendations were not universally implemented (particularly in Canada and Germany). The exceptions were the UK, which has a very centralized immunization program, and Australia, which was decentralized but took a more centralized approach to monitoring and promotion.
Fig. 3.
Perceived capability of national adult vaccine implementation, by case study country. Respondents rated their agreement or disagreement with the seven listed statements on their perceptions of adult vaccine implementation in their country. Scores were: (0) unclear or no comment (1) strongly disagree (2) somewhat agree (3) strongly agree.
Access to immunization was a factor reported to influence older adult immunization uptake. Lower uptake may be due to limited mobility or lack of awareness of the need for adult vaccines. In some countries, such as Germany and Japan, respondents stated that vaccines were still viewed as something only for children. Additionally, health system complexity was reported as an important factor contributing to access, and ultimately uptake, with some respondents describing receiving vaccination as an older adult as an overly cumbersome process. In Canada, respondents’ general perception was that there are not many places or providers of adult vaccination. Restrictions on who could administer vaccines was also reported as a perceived barrier. Improvements in access were described in Japan, where nurses can now vaccinate; in France, where pharmacists can offer influenza vaccines; and in the UK, where pharmacists were allowed to administer influenza and pneumococcal vaccines.
Despite some countries expanding their range of adult vaccine providers, respondents noted that uptake does not always correspondingly increase. This gap offers a role for advocacy efforts in improving uptake. The influence of advocacy can be difficult to assess and is sometimes subjective. Nonetheless, we used the number of national advocacy organizations as a proxy for influence in database countries. In case countries, we were able to gather insights.
Each respondent was asked about advocacy efforts in their country, yet there was a wide range of level of familiarity of country efforts. Australia is a clear leader and uses a variety of approaches to advocate for adult immunization. Australian respondents described influential advocacy efforts that impacted both the national adult vaccination decision-making and implementation processes. For example, in the country citizens, government, medical societies, and health aging specialists have organized large groups to advocate for better recommendations and financing as well as influence uptake and equal access to vaccines. In Japan, respondents were not familiar with any major advocacy effort, although could describe small-scale patient-advocacy groups or activism against the government regarding vaccine injuries.
3.2. Indicators of decision-making and implementation and uptake to map performance
Based on the findings from the case studies, ten indicators emerged as descriptors of countries’ older adult immunization decision-making capacity, specifically whether countries: were early adopters; had country-specific barriers such as requirements for technology transfer (Brazil, India, Japan, Korea); preferences for indigenous product (China, Indonesia, Brazil, India, Russia); halal vaccines (Malaysia); safety study requirements (Japan); or C-E requirements (UK, Netherlands); prioritized surveillance on VPDs in older adults (emerging as a factor in many of the countries concerned about health security); were prompted in their vaccine decisions by health security concerns; considered C-E a major driver in vaccine decisions; had working groups in their National Immunization Technical Advisory Groups (NITAGs) specific to adult vaccine issues; adopted Pneumococcal conjugate vaccine (PCV)1 ; adopted Herpes Zoster vaccine (HZV); published a national healthy aging policy (including whether there is a mention of vaccines); and included adult vaccines in their national immunization policy.
For implementation and uptake, indicators that emerged included whether there was government financing for influenza vaccines, PCV and HZV; centralized vaccine registries for children and adults; coverage data for all three vaccines; advocacy (measured by the number of advocacy organizations); documented influence of organizations or leaders; access (easy to get vaccinated by expanded list of providers or lack of bureaucracy or cumbersome process); equity focus; centralization of the adult vaccine program; and centralization of the health system.
Few countries that had strong decision-making also had strong uptake and vice-versa. Further, the scoring table shows that countries varied significantly in the indicators that drove their performance on adult vaccine decision-making (Table 5 ) and implementation (Table 6 ). To determine how composite scores corresponded to performance, we ordered countries based on their pneumococcal vaccine uptake and compared that to rankings of the composite score on both decision-making and implementation/uptake. Countries with the highest coverage of pneumococcal vaccines did not always perform the highest on decision-making, with the exception of Australia, US, UK, and Canada. We did a similar exercise for influenza vaccine uptake and found that top ten countries with highest influenza coverage were similar to those with the highest decision-making and implementation scores.
Table 5.
Country Scores on Robustness of Decision-Making.
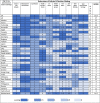 |
See Table 2 for score descriptors. Generally, the higher the score the better that country meets the indicator; 0 scores either mean the country doesn't meet the indicator or no data were found.
Table 6.
Country Scores on Implementation.
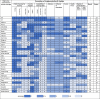 |
See Table 2 for score descriptors. Generally, the higher the score the better that country meets the indicator; 0 scores either mean the country doesn't meet the indicator or no data were found.
The US had the highest ranking, compared to other countries, and scored 17 points when assessed for the robustness of policies and decision-making. Australia (score: 16.5), the UK (score: 16.5) and Italy (score: 16), had the next set of highest scores. Countries with the least robust policies and decision-making were Denmark, India, Peru, the Philippines, Switzerland, and Saudi Arabia (all ranking last; scoring: 4) (see Table 5). When assessed for the promotion of implementation and uptake, the UK (score: 23 out of a possible 28), New Zealand (22), and Australia (21) had the highest rankings compared to other countries. In contrast, Russia (score: 4), Peru (4), and India (5) had the lowest ranking (see Table 6).
3.3. Archetypes
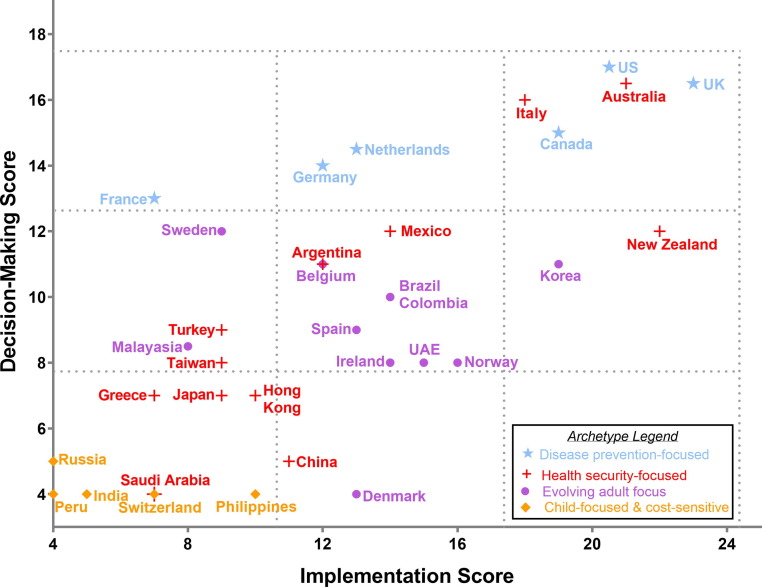
Based on a synthesis of case study data, we found four distinct archetypes with unique characteristics: “disease prevention-focused”; “health security-focused”; “evolving adult focus”; and “child-focused and cost-sensitive” (Fig. 4 ). Some countries could belong to multiple archetypes, but we selected the archetype most closely aligned with primary driver of approach.
Fig. 4.
Country Score Plot and Adult Vaccine Archetypes. Combining two separate analyses, this graph plots countries’ performance in implementation and decision-making as a point estimate, overlaid with each country’s primary archetype denoted by a symbol (within an archetype, countries share similarities around vaccine decision-making; (see Table 5). Countries are plotted according to their adult vaccine implementation score (see Table 5) on the x axis and their adult vaccine decision-making score (see Table 6) on the y axis. For example, the UK scored 23 in implementation and 16.5 in decision-making, and is plotted at (23, 16.5). Some countries share the same coordinate, with two names next to a single point. Countries with the highest implementation and decision-making scores appear in the top right corner of the graph. Primary archetype (disease prevention focused; health security focused; evolving adult focus; or cost-sensitive and child focused) is designated by the color of the country’s name and the symbol overlaid each point. Most countries (n=12) belong to the health security primary archetype.
We also noted that the highest performing countries in terms of both decision-making (Australia, US, UK, Canada, Italy, Netherlands, Germany and Mexico) and implementation (UK, New Zealand), Australia, US, Korea, Canada, Italy and Norway) belonged to either the “disease prevention-focused” or “health security-focused” archetype (see Fig. 4).
Disease prevention-focused: Countries in the “disease prevention” archetype included Canada, France, Germany, Netherlands, UK, and US. These countries valued the use of data and process. Most countries’ NITAGs in this archetype had considered most of the adult vaccines and performed fairly high on decision-making. We noted use of their own disease burden/impact evidence in decision-making, as well as use of other countries’ data. Most of these countries had their own adult surveillance and formal adult vaccine working groups on their NITAG. The UK and the Netherlands also placed high importance on economics. Most countries considered economics, but it was not a primary driver. Germany, and to a lesser extent the US, considered economics, but disease burden was the primary driver in decisions. There was significant variation in implementation performance. Reasons varied within this archetype, and included lack of national adult registries, equity focus, sufficient advocacy and centralization.
Health security-focused: The “health security” archetype was the largest of the four and also the most diverse in terms of performance. It included Argentina, Australia, China, Greece, Hong Kong, Italy, Japan, Mexico, New Zealand, Saudi Arabia, Taiwan, and Turkey. The characteristic of this archetype is that outbreaks (H1N1 in Australia and Argentina; pneumonia in Japan), VPD threats (due to migration or in refugees), and natural disasters (tsunami in Japan) were viewed as an important country motivation for action. We saw wide variation of performance within this archetype, with Australia, Italy, New Zealand and Mexico performing highest and China, Japan, Hong Kong, Taiwan and Greece lagging behind. Degree of centralization and registries contributed to performance. Australia, for example, reported that data use was strengthened during the H1N1 outbreak in 2009. In Argentina, surveillance and epidemiologic response was also strengthened as a result of an H1N1 outbreak. It also led to strategies to establish mass vaccination centers to improve access to vaccines more widely. In 2011, the Great East Japan Earthquake struck north-eastern Japan and destroyed the local healthcare system. At the time, pneumococcal vaccine coverage in Japan was only 11% and many healthcare workers feared the occurrence of pneumonia outbreak at evacuation shelters [39]. To avoid future outbreaks, pneumococcal vaccine was provided free of charge in the three prefectures most affected by the earthquake. In 2013, they reached record levels of uptake with Miyagi and Iwate at 45%, and Fukushima at 60% coverage [39], leading to significant reduction in deaths attributed to pneumonia in the area [40], and eventually to the Ministry of Health, Labour, and Welfare’s decision in 2014 to include pneumococcal vaccine to the routine immunization schedule for adults aged 65 and older. The national coverage increased from 11% in 2011 to 28% in 2016 [41].
Evolving adult focus: Countries in the “evolving adult focus” archetype include Belgium, Brazil, Colombia, Ireland, Korea, Spain, Sweden and Malaysia. These countries had moderate to strong systems and decision-making ranged from weak (Denmark, Malaysia) to moderate (Korea, Belgium). Many of the countries in this archetype lack a strong NITAG for adult vaccine decisions, but exhibit some elements of the “disease prevention” archetype. Some have healthy aging policies or immunization strategies, but only Brazil has both. Some countries were early adopters for some adult vaccines, including PCV in Ireland and Belgium, and HZ vaccine in UAE and Norway. Belgium has published an adult immunization strategy recently [42]. Financing for recommended adult vaccines, varies as well. Although vaccines were recommended in some countries, they were not always publicly financed or financed for risk groups.
Child-focused and cost-sensitive: Finally, some countries, including Russia, Peru, India, Switzerland and the Philippines remain “child-focused and cost-sensitive” in their public markets. The countries in this archetype have not prioritized adult immunization programs and have generally lacked focus on decision-making for older adults. We found no adult vaccine working groups on the three vaccines analyzed (influenza, pneumococcal and herpes zoster) or policies around adult immunization at the time of our study. In terms of implementation, these countries may require patients to pay out of pocket for adult vaccines (Russia, India) although there were some exceptions for influenza vaccine like the Philippines and Switzerland (although in Switzerland vaccines were covered through insurance). We did not find a focus on implementation. These five countries have no registries or policies around adult health or adult immunization. Additionally, advocacy for adult immunization was not strong and, in most cases, seemed to have little influence on the outcomes of the government. Lastly, for most countries in this archetype, there is a cost-based argument: given limited resources, public investments in child health and vaccines are prioritized, as they are seen as necessary to further national economic development (Russia, Peru, India, Philippines).
3.4. Validation
We were able to validate our data and scoring in 21 of 34 countries (62%) (Table 3). Where there was disagreement and documented sources, we corrected data and in five instances, adjusted scoring to reflect a more accurate picture of the parameters which had to do with timing of decisions or policies, the importance of C-E data in decision-making, NITAG working groups, and a mixed financing system.
3.5. Limitations
Importantly, the act of classifying countries into a primary archetype has its own set of limitations. Firstly, there is a level of subjectivity that comes with archetyping, which we have tried to ameliorate by taking an evidence-based approach. Secondly, archetyping elevates a certain set of common characteristics above others and there are variations between and within countries that get under-accounted. Countries may not fit perfectly in an archetype and may actually belong to other archetypes simultaneously; we therefore classified countries into primary archetypes, according to the indicator that they scored the highest on.
Our analysis was also limited by data availability and quality. In some cases, a zero score was given due to a lack of data or clarity on an indicator. We tried to address this through the validation process, but little additional information was provided. Additionally, each indicator’s scoring was based only on data that was publicly available through October 2018 (unless otherwise provided by experts during validation) and may be impacted by timing. Changes in scoring of 1–2 points is unlikely to impact score mapping, but larger changes may shift archetype categorization.
4. Discussion
Archetypes can be useful in identifying factors that are most influential in improving decision-making and implementation for adult vaccines in the context of a country’s approach and priorities. This will enable various countries to learn from experiences amongst countries within the same archetype. There has been some use of archetypes, mainly in health technology assessment [41], [42], [43], [44], [45], but experiences have not yet been widely documented.
The archetype describes the primary driver of decisions and implementation as well as country priorities or approach rather than performance. There can be significant variation in performance within an archetype, which we saw in this exercise. Top performing countries belonged to two different archetypes, which were “disease prevention-focused” (US, UK, Canada) and “health security-focused” (Australia, Italy), suggesting that there are multiple ways to achieve adoption and uptake. It is likely that countries within the “evolving adult focus” archetype could improve their performance by learning from countries in the “disease prevention” or “health security” archetypes. The improvements needed within the “child-focused” archetype may require both stronger advocacy as well as additional data. While we provide broad-based archetypes, we also saw different approaches within the archetypes; this is important as each country will need to take lessons based on what works for them. Understanding the success factors of the highest performing countries within a particular domain (e.g., decision-making) can also help other countries move up their performance in the context of their structure and priorities.
The countries in the “disease prevention” archetype all value data and its use, albeit to different levels and strategies can focus on data use and advocacy for disease prevention. At the one extreme of this archetype is the UK, where data use permeates through the process of both decision-making and implementation. Decisions are evidence-based, with little influence of advocacy or champions. On the implementation side, there was also strong use of data with information on the immunization status of individual patients, which is all mapped to their general practitioner (GP). For countries who will consider a centralized approach, the UK provides a good model. However, many countries have decentralized systems, including the US and Canada, who also have strong use of data for decision-making. They also have greater degrees of advocacy, which could perhaps also ensure certain groups have a voice in the process. Use of data for implementation in decentralized settings may also benefit from learnings from countries that have centralized registries in a decentralized system (e.g., Australia, although a different archetype).
Health security concerns provide countries with an opportunity to overcome a wide array of issues, from financing to barriers that may slow down decisions (e.g., tech transfer requirements as seen in Brazil or requirements/strong preference for local products such as in China or India, requirements for local studies as in Japan, etc.) and may be a motivator to strengthen surveillance, use of data, communications and platforms. The current context of COVID-19 highlights likely opportunities to leverage the importance of developing a strong system. In doing so, it is important for countries to consider how all people, including older adults and/or marginalized populations such as migrants or refugees, can access vaccines. For example, in the US, adult immunization access increased as a result of the 2009 influenza An H1N1 pandemic, with states granting pharmacists the authority to vaccinate against influenza [46]. Also, during that pandemic, a study demonstrated that American Indian and Alaska Native persons have a higher risk for death from the H1N1 influenza [47] supporting prioritization of seasonal influenza vaccines amongst native Americans that year [48]. We saw wide variation of performance within this archetype and note that while emergencies may provide motivation to take action, other contextual factors must be taken into account. Degree of centralization of adult immunization is one important factor that influences success of implementation. Registries and stronger use of data are an important element in both “health security” and “disease prevention” archetypes, but the motivator to get them done may be different – in times of emergency, data are essential, but it is prior to an emergency that data are needed [52]. After the 2003 severe acute respiratory syndrome (SARS) epidemic, China was challenged to improve its public health emergency management systems (PHEMS) [49]. The system was significantly improved within ten years after the outbreak [49]. More recently, China was able to contain COVID-19 within the country [50], but the virus has caused more than 1.2 million cases and 70,000 deaths worldwide and continues to spread as of early April [51]. There is now more than ever a strong need for systems to detect risk, transparently communicate the risk, and have infrastructure in place to address both existing and emerging infectious threats[48]. Engaging civil society and building accountability mechanisms can help motivate action more urgently. This current pandemic provides a window of opportunity to build urgency for the need for adult immunization and systems to deliver them. Efforts, however, must not focus solely on COVID-19, but on the platforms needed to build strong health systems for all ages, including older adults. In countries that have taken action in the face of an emergency this message will resonate; for others, particularly without the resources or capacity to address older adults, different approaches may be needed to build focus on the needs of this group.
In countries with an evolving adult focus, a strategy could be to emphasize NITAG strengthening and sharing experiences with the “disease prevention” and/or “health security” archetypes. This will be particularly important should a vaccine become available for COVID-19 and in considering vaccines that can address VPDs in an older population now. In countries who follow other countries’ recommendations, stronger global guidance is needed to emphasize preparedness, highlight the importance a growing adult population, and the consequences of doing nothing. In countries that need to improve uptake, access, financing, registries, and monitoring are all important. One factor that correlates to uptake is expansion through pharmacists [54], although with COVID-19, perhaps other ways of delivery that don’t require contact with people could be considered. Additional provider-focused strategies could be implemented to improve uptake of current vaccines, such as financial incentives to vaccinate older adult patients. Proactive outreach (e.g., actively sending reminders to patients to get their vaccines) may also be important post-pandemic to remind patients of vaccines’ importance.
Finally, even in the “child-focused and cost-sensitive” archetype there may be opportunities to take advantage of synergies and address immunization through broader issues such as universal health coverage and antimicrobial resistance. Central to these opportunities is building the links between child and adult health, and economic development [55]. The economic pressure placed on countries through COVID-19 may make adult immunization seem unreachable and studies that capture the broader value of vaccines may help in justifying investments[16], [20]. Stronger global guidance synthesizing healthy aging, universal health coverage, antimicrobial resistance and immunization recommendations and agendas can also be helpful. Importantly, strong individual champions, coalition building across the vaccine and aging communities, and more resonant messaging could all help elevate routine older adult vaccination as a priority [56]. A critical, momentum-building milestone would be when global institutions, like WHO, commit to leading the coordination of implementation [56], empowering countries to think about implementing older adult immunization in a more timely manner.
4.1. Implications of vaccine confidence and hesitancy
Within and across all four archetypes, vaccine confidence plays a role as both a facilitator and barrier of countries’ performance. Although fear of side effects, spread of misinformation, lack of provider recommendation, and reduced belief that vaccines are valuable are common reasons for hesitancy across all ages, a recent review of the literature yielded few insights into describing how adult vaccine hesitancy varied from that for children [57]. This is an area of further research that may help explain country uptake and better address implementation gaps. Specifically, once the research gap is more clearly described, tailored communication and outreach strategies could be developed that encourage older adults to seek immunization services.
5. Conclusion
Countries take different approaches to adult immunization. Drivers and facilitators of primary adult immunization archetypes should be considered when developing global guidance for countries. Experiences and lessons learned should be shared within archetypes to improve performance of countries falling behind on decision-making or implementation. The results of this study may inform strategies in countries with similar contexts and priorities. Further research may be beneficial to further refine archetypes and expand the understanding of what influences success within an archetype. This can help advance policies and action that will improve vaccine access for older adults and build a stronger appreciation of the value of immunization amongst a variety of stakeholders.
CRediT authorship contribution statement
Lois Privor-Dumm: Supervision, Conceptualization, Methodology, Investigation, Writing - original draft, Writing - review & editing, Supervision, Conceptualization, Methodology, Investigation, Writing - original draft, Writing - review & editing. Prarthana Vasudevan: Data curation, Investigation, Validation, Visualization, Writing - review & editing. Kana Kobayashi: Data curation, Validation, Writing - review & editing. Jaya Gupta: Methodology, Writing- review & editing.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: LPD has received grants from GlaxoSmithKline, Merck and Pfizer and an honorarium from Pfizer. The other authors declare no competing interests.
Acknowledgements
GlaxoSmithKline (GSK) Biologicals, Belgium provided funding for the archetype analysis. Merck and GSK provided funding for the landscape analysis and case studies. The authors thank Nina Martin, Geervani Daggupati, Nobutoshi Nawa, So Yoon Sim, Dexter Waters, and Gatien de Broucker, for their valuable contributions; members of the International Council on Adult Immunization (ICAI) for their review and input; the in-country experts who participated in case study interviews and provided input in the validation process; and Haley Budigan for her careful proofreading.
Footnotes
PCV was selected rather than pneumococcal polysaccharide vaccine (PPSV) to differentiate countries. Most countries had already adopted PPSV. PCV was considered “adopted” if used alone or sequentially following PPSV and included countries with risk group recommendations only.
References
- 1.Beard J.R. The World report on ageing and health: a policy framework for healthy ageing. Lancet. 2016;387(10033):2145–2154. doi: 10.1016/S0140-6736(15)00516-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.GBD 2017 DALY and Hale Collaborators. Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392(10159):1859–1922. [DOI] [PMC free article] [PubMed]
- 3.McLaughlin J.M. Estimated Human and Economic Burden of Four Major Adult Vaccine-Preventable Diseases in the United States, 2013. J Prim Prev. 2015;36(4):259–273. doi: 10.1007/s10935-015-0394-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McElhaney J.E. T-Cell Immunity to Influenza in Older Adults: A Pathophysiological Framework for Development of More Effective Vaccines. Front Immunol. 2016;7:41. doi: 10.3389/fimmu.2016.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson R.W. Herpes zoster epidemiology, management, and disease and economic burden in Europe: a multidisciplinary perspective. Ther Adv Vacc. 2015;3(4):109–120. doi: 10.1177/2051013615599151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leidner A.J. Cost-effectiveness of adult vaccinations: A systematic review. Vaccine. 2019;37(2):226–234. doi: 10.1016/j.vaccine.2018.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ozawa S. Modeling The Economic Burden Of Adult Vaccine-Preventable Diseases In The United States. Health Aff (Millwood) 2016;35(11):2124–2132. doi: 10.1377/hlthaff.2016.0462. [DOI] [PubMed] [Google Scholar]
- 8.Wateska A.R. Cost-effectiveness of adult pneumococcal vaccination policies in underserved minorities aged 50–64years compared to the US general population. Vaccine. 2019;37(14):2026–2033. doi: 10.1016/j.vaccine.2019.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Friesen K.J. Cost of shingles: population based burden of disease analysis of herpes zoster and postherpetic neuralgia. BMC Infect Dis. 2017;17(1):69. doi: 10.1186/s12879-017-2185-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dabestani N.M. A review of the cost-effectiveness of adult influenza vaccination and other preventive services. Prev Med. 2019;126:105734. doi: 10.1016/j.ypmed.2019.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.United Nations Department of Economic and Social Affairs Population Division. World Population Prospects 2019: Highlights; 2019.
- 12.European Commission. In Sobczak D, editor. Population ageing in Europe. Facts, implications and policies. Brussels: European Commission Directorate‐General for Research and Innovation Socioeconomic sciences and humanities; 2014.
- 13.Doherty T.M., Del Giudice G., Maggi S. Adult vaccination as part of a healthy lifestyle: moving from medical intervention to health promotion. Ann Med. 2019;51(2):128–140. doi: 10.1080/07853890.2019.1588470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weinberger B. Vaccines for the elderly: current use and future challenges. Immun Ageing. 2018;15:3. doi: 10.1186/s12979-017-0107-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Esposito S. The public health value of vaccination for seniors in Europe. Vaccine. 2018;36(19):2523–2528. doi: 10.1016/j.vaccine.2018.03.053. [DOI] [PubMed] [Google Scholar]
- 16.Bloom D.E. Moving beyond traditional valuation of vaccination: Needs and opportunities. Vaccine. 2017;35(Suppl 1):A29–A35. doi: 10.1016/j.vaccine.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 17.Andrew M.K. Influenza Vaccination in Older Adults: Recent Innovations and Practical Applications. Drugs Aging. 2019;36(1):29–37. doi: 10.1007/s40266-018-0597-4. [DOI] [PubMed] [Google Scholar]
- 18.Mulpuru S. Effectiveness of Influenza Vaccination on Hospitalizations and Risk Factors for Severe Outcomes in Hospitalized Patients With COPD. Chest. 2019;155(1):69–78. doi: 10.1016/j.chest.2018.10.044. [DOI] [PubMed] [Google Scholar]
- 19.Teresa Aguado M. Report on WHO meeting on immunization in older adults: Geneva, Switzerland, 22–23 March 2017. Vaccine. 2018;36(7):921–931. doi: 10.1016/j.vaccine.2017.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cafiero-Fonseca E.T. The full benefits of adult pneumococcal vaccination: A systematic review. PLoS ONE. 2017;12(10):e0186903. doi: 10.1371/journal.pone.0186903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rehm S.J. Identifying barriers to adult pneumococcal vaccination: an NFID task force meeting. Postgrad Med. 2012;124(3):71–79. doi: 10.3810/pgm.2012.05.2550. [DOI] [PubMed] [Google Scholar]
- 22.Tan L. Adult vaccination: Now is the time to realize an unfulfilled potential. Hum Vaccin Immunother. 2015;11(9):2158–2166. doi: 10.4161/21645515.2014.982998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National Vaccine Advisory C. A pathway to leadership for adult immunization: recommendations of the National Vaccine Advisory Committee: approved by the National Vaccine Advisory Committee on June 14, 2011. Public Health Rep 2012;127(Suppl 1):1–42. [DOI] [PMC free article] [PubMed]
- 24.Bonanni P. Focusing on the implementation of 21st century vaccines for adults. Vaccine. 2018;36(36):5358–5365. doi: 10.1016/j.vaccine.2017.07.100. [DOI] [PubMed] [Google Scholar]
- 25.Esposito S. Recommended immunization schedules for adults: Clinical practice guidelines by the Escmid Vaccine Study Group (EVASG), European Geriatric Medicine Society (EUGMS) and the World Association for Infectious Diseases and Immunological Disorders (WAidid) Hum Vaccin Immunother. 2016;12(7):1777–1794. doi: 10.1080/21645515.2016.1150396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gutierrez-Robledo L.M. First Mexican Consensus of Vaccination in Adults. Gac Med Mex. 2017;153(Suppl 1):5. [PubMed] [Google Scholar]
- 27.SAATI Partnership. Supporting Active Ageing Through Immunisation Partnership. Adult vaccination: a key component of healthy ageing – The benefits of life-course immunisation in Europe; 2013.
- 28.Ortiz J.R. A global review of national influenza immunization policies: Analysis of the 2014 WHO/UNICEF Joint Reporting Form on immunization. Vaccine. 2016;34(45):5400–5405. doi: 10.1016/j.vaccine.2016.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu L.A. Adult immunization policies in advanced economies: vaccination recommendations, financing, and vaccination coverage. Int J Public Health. 2013;58(6):865–874. doi: 10.1007/s00038-012-0438-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kanitz E.E. Variation in adult vaccination policies across Europe: an overview from VENICE network on vaccine recommendations, funding and coverage. Vaccine. 2012;30(35):5222–5228. doi: 10.1016/j.vaccine.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 31.de Gomensoro E., Del Giudice G., Doherty T.M. Challenges in adult vaccination. Ann Med. 2018;50(3):181–192. doi: 10.1080/07853890.2017.1417632. [DOI] [PubMed] [Google Scholar]
- 32.Menzies R.I. Vaccine myopia: adult vaccination also needs attention. Med J Aust. 2017;206(6):238–239. doi: 10.5694/mja16.00811. [DOI] [PubMed] [Google Scholar]
- 33.Webster F. What isn't measured isn't done - eight years with no progress in Aboriginal and Torres Strait Islander adult influenza and pneumococcal vaccination. Aust N Z J Public Health. 2019;43(6):558–562. doi: 10.1111/1753-6405.12944. [DOI] [PubMed] [Google Scholar]
- 34.Harris K.M. A Blueprint for Improving the Promotion and Delivery of Adult Vaccination in the United States. Rand Health Q. 2012;2(1):15. [PMC free article] [PubMed] [Google Scholar]
- 35.The World Health Organization. The Global Vaccine Action Plan 2011–2020. Geneva; 2013.
- 36.The World Health Organization. Immunization Agenda 2030: A Global Strategy To Leave No One Behind. 2019 January 6, 2020]; SAGE Submission. Available from: https://www.who.int/immunization/sage/meetings/2019/october/2_ia2030_SAGE_submission.pdf?ua=1.
- 37.Ozawa S. Evidence-to-policy gap on hepatitis A vaccine adoption in 6 countries: Literature vs. policymakers' beliefs. Vaccine. 2014;32(32):4089–4096. doi: 10.1016/j.vaccine.2014.05.026. [DOI] [PubMed] [Google Scholar]
- 38.Sauer M, V.P., Meghani A, Luthra A, Garcia C, Knoll M, Privor-Dumm L, Situational assessment of adult vaccine preventable disease and the potential for immunization advocacy and policy in low- and middle-income countries 2020 [in press]. [DOI] [PMC free article] [PubMed]
- 39.Naito T. An activity report of physicians of the Department of General Medicine, Juntendo University School of Medicine against the Great East Japan Earthquake. J Gen Family Med. 2015;38:123–124. [Google Scholar]
- 40.Naito T. Relationship between public subsidies and vaccination rates with the 23-valent pneumococcal vaccine in elderly persons, including the influence of the free vaccination campaign after the Great East Japan Earthquake. J Infect Chemother. 2014;20(7):450–453. doi: 10.1016/j.jiac.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 41.Ministy of Health, Labour, and Welfare. Pneumococcal vaccine (PPSV23); 2018.
- 42.Superior Health Council Belgium. Advies 9141 – Basisvaccinatieschema; 2019; Available from: https://www.health.belgium.be/en/node/35437#anchor-35437.
- 43.Allen N. Development of archetypes for non-ranking classification and comparison of European National Health Technology Assessment systems. Health Policy. 2013;113(3):305–312. doi: 10.1016/j.healthpol.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 44.Feifer C. Different paths to high-quality care: three archetypes of top-performing practice sites. Ann Fam Med. 2007;5(3):233–241. doi: 10.1370/afm.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mazumder D. Segmentation of Seven Asia-Pacific Health Technology Assessment (HTA) Agencies into Different Evolutionary HTA Archetypes. Value Health. 2016;19(7):A832. [Google Scholar]
- 46.Fitzgerald T.J. Integrating pharmacies into public health program planning for pandemic influenza vaccine response. Vaccine. 2016;34(46):5643–5648. doi: 10.1016/j.vaccine.2016.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.CDC. Deaths Related to 2009 Pandemic Influenza A (H1N1) Among American Indian/Alaska Natives - 12 states; 2009. [PubMed]
- 48.CDC. Prevention and Control of Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices (ACIP); 2010. [PubMed]
- 49.Sun M. The public health emergency management system in China: trends from 2002 to 2012. BMC Public Health. 2018;18(1):474. doi: 10.1186/s12889-018-5284-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ng Y. Evaluation of the Effectiveness of Surveillance and Containment Measures for the First 100 Patients with COVID-19 in Singapore - January 2-February 29, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(11):307–311. doi: 10.15585/mmwr.mm6911e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Johns Hopkins University and Medicine. Coronavirus Resource Center; 2020 [cited 2020 April 5]; Available from: https://coronavirus.jhu.edu/map.html.
- 52.Vong S., O'Leary M., Feng Z. Early response to the emergence of influenza A(H7N9) virus in humans in China: the central role of prompt information sharing and public communication. Bull World Health Organ. 2014;92(4):303–308. doi: 10.2471/BLT.13.125989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lancet The. COVID-19: too little, too late? Lancet. 2020;395(10226):755. doi: 10.1016/S0140-6736(20)30522-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ecarnot F. Pharmacy-based interventions to increase vaccine uptake: report of a multidisciplinary stakeholders meeting. BMC Public Health. 2019;19(1):1698. doi: 10.1186/s12889-019-8044-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Privor-Dumm L. Life-course immunization: Building the consensus for adult vaccination in IFPMA Thought Leader Series; 2019.
- 56.Shiffman J., Smith S. Generation of political priority for global health initiatives: a framework and case study of maternal mortality. Lancet. 2007;370(9595):1370–1379. doi: 10.1016/S0140-6736(07)61579-7. [DOI] [PubMed] [Google Scholar]
- 57.Cull A. Characterizing Global Vaccine Hesitancy: How Adult and Pediatric Hesitancy May Differ. Johns Hopkins Bloomberg School of Public Health; 2019.