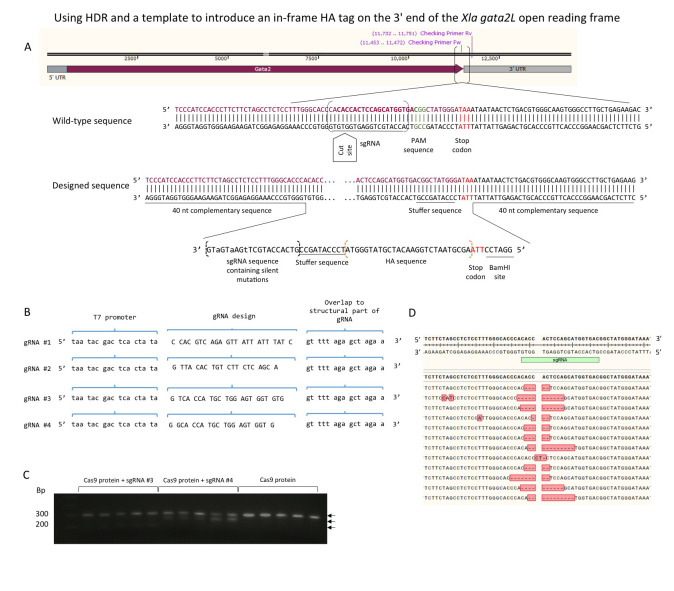
Figure 1.
Targeted insertion of an HA tag into the 3’ end of Xenopus laevis gata2.L. Four single guide RNA (sgRNA) sequences were tested for efficiency by T7 assay before designing the ssOligonucleotide (A) containing the HA tag around the most efficient sgRNA (sgRNA #4, B). The 146 nucleotides long ssDNA fragment contained forty base complementary regions flanking the cutting site containing silent mutations to prevent re-cutting, an HA tag, a stop codon and a BamHI site, to allow the identification of positive clones. Amplification with forward (5’ ACTCCCATCCACCCTTCTTC 3’) and reverse (5’ CGATTGCTGTGTCCGACTTT 3’) primers, followed by digestion of the target region with BamHI would cut the target sequence containing the insertion into two smaller bands that could later be visualised by agarose gel electrophoresis. This approach was not successful. We hypothesise that the mosaicism in F0 animals subjected to PCR reduced the possibility of successfully visualising the digested product. Two primers extending from the HA tag into the genome were designed (HA Fw Primer 5’ CCCATACGATGTTCCAGATTACGC 3’; HA Rv Primer 5’ GCGTAATCTGGAACATCGTATGGG 3’) to selectively amplify the HA insertion successfully discerning between animals containing the insertion and those not. (C.) Five embryo from each experimental condition and five control embryos were analysed and T7 endonuclease treated amplicons were visualized via gel electrophoresis, sub-cloned into pGemTeasy and sequenced (D). Eighty-one percent of the sequences obtained showed deletions around the desired cut site and 6 % contained random insertions.