Abstract
Type 2 diabetes (T2D) is associated with an increased risk of cardiovascular disease and heart failure, which highlights the need for improved understanding of factors contributing to the pathophysiology of these complications as they are the leading cause of mortality in T2D. Patients with T2D have high levels of epicardial adipose tissue (EAT). EAT is known to secrete inflammatory factors, lipid metabolites, and has been proposed to apply mechanical stress on the cardiac muscle that may accelerate atherosclerosis, cardiac remodeling, and heart failure. High levels of EAT in patients with T2D have been associated with atherosclerosis, diastolic dysfunction, and incident cardiovascular events, and this fat depot has been suggested as an important link coupling diabetes, obesity, and cardiovascular disease. Despite this, the predictive potential of EAT in general, and in patients with diabetes, is yet to be established, and, up until now, the clinical relevance of EAT is therefore limited. Should this link be established, importantly, studies show that this fat depot can be modified both by pharmacological and lifestyle interventions. In this review, we first introduce the role of adipose tissue in T2D and present mechanisms involved in the pathophysiology of EAT and pericardial adipose tissue (PAT) in general, and in patients with T2D. Next, we summarize the evidence that these fat depots are elevated in patients with T2D, and discuss whether they might drive the high cardiometabolic risk in patients with T2D. Finally, we discuss the clinical potential of cardiac adipose tissues, address means to target this depot, and briefly touch upon underlying mechanisms and future research questions.
Keywords: epicardial adipose tissue, type 2 diabetes, cardiovascular disease, cardiac adipose tissue, pericardial adipose tissue
Introduction
Diabetes is one of the fastest-growing health challenges, with 463 million diagnosed today and rising to an estimated 700 million by 2045.1 Type 2 diabetes (T2D) is by far the most prevalent subtype, accounting for 90% of all cases of diabetes.1 Patients with T2D have 2- to 4-fold increased risk of cardiovascular disease (CVD) and heart failure,2–6 and, in 2019, more than 4 million people died globally from diabetes-related complications,7 namely CVD and heart failure.8–11 In order to prevent these premature deaths, there is a need for improved understanding of the pathophysiology, and thereby identification, of novel risk factors that can aid early detection of high-risk patients and aggressive treatments.
Obesity is one of the important risk factors driving the increased rate of CVD and heart failure in T2D due to, for example, altered hemodynamic load, neurohumoral activation, cardiac metabolism, adipokine secretion, and low-grade inflammation.12–19 Traditionally, obesity [defined as body mass index (BMI) > 30 kg/m2] per se has been viewed as a risk factor, but it is now recognized that fat depots are heterogenous; they differ in their lipolytic activity, insulin sensitivity, secretory capacity and location, and, thus, in their atherogenic potential.15,16,18,20–24 This recognition has shaped the idea that it is primarily the visceral fat tissues located adjacent to the coronary arteries and the myocardium, the epicardial adipose tissue (EAT) and pericardial adipose tissue (PAT), that accelerate coronary atherosclerosis and myocardial dysfunction due to their lipolytic and secretory hyperactivity leading to accumulation of toxic lipid metabolites in the myocardium and endothelium. Since T2D is accompanied by an expansion of EAT and PAT,25 these depots have been suggested to play a critical role in accelerating CVD and heart failure, particularly in patients with T2D.26–30 In support of this, high levels of EAT in T2D have been associated with atherosclerosis,31 diastolic dysfunction,32 and incident cardiovascular events.33
In this review, we outline the evidence that EAT acts as a link coupling diabetes and CVD. First, we present the pathophysiological mechanisms of EAT and PAT. Next, we account for the role of EAT in T2D, and, finally, we discuss the clinical potential of EAT in cardiovascular risk assessment and prevention, including how it can be targeted, and highlight future research questions.
Mechanisms of epicardial adipose tissue and cardiovascular pathophysiology
Anatomical characteristics of EAT affecting pathophysiology
Human EAT comprises adipocytes, stromo-vascular cells, neurons, and immune cells.34–36 Several characteristics related to the anatomy of EAT suggest that this depot may play a particularly important role in T2D and cardiovascular physiology and pathophysiology. First, since no fascia separates the tissues, EAT is in direct contact with the myocardium, allowing direct communication.37–39 Second, EAT and the myocardium share microcirculation, enabling vasocrine crosstalk.34,35,40 Third, despite the fact that EAT is associated mostly with the free wall of the right ventricle, the atrioventricular grooves, the apex, and the coronary arteries, it can cover up to 80% of the surface of the heart.34,41 Consequently, it is possible that EAT affects the circulation of the coronary artery and the myocardial diastolic and systolic properties mechanically.
Metabolic characteristics of EAT
For the major part of the 20th century, EAT was considered an unimportant inert supporting structure and energy depot of the heart, and attracted no attention apart from sporadic scientific papers that hinted at an active metabolic role.42 However, in 1989, Marchington et al. showed that lipolysis and fatty acid synthesis are greater in EAT compared with visceral fat (VAT) and other cardiac fat tissue (the PAT).41 This finding demonstrated that EAT is metabolically very active, which fitted with the finding that EAT adipocytes are smaller than other VAT cells.34 The appreciation of EAT as a metabolically active tissue motivated hypotheses of EAT being an important source of energy for the myocardium during periods of increased energy demand, and for being able to regulate free fatty acids levels in the coronary arteries and the accumulation of toxic lipid levels in cardiomyocytes.41
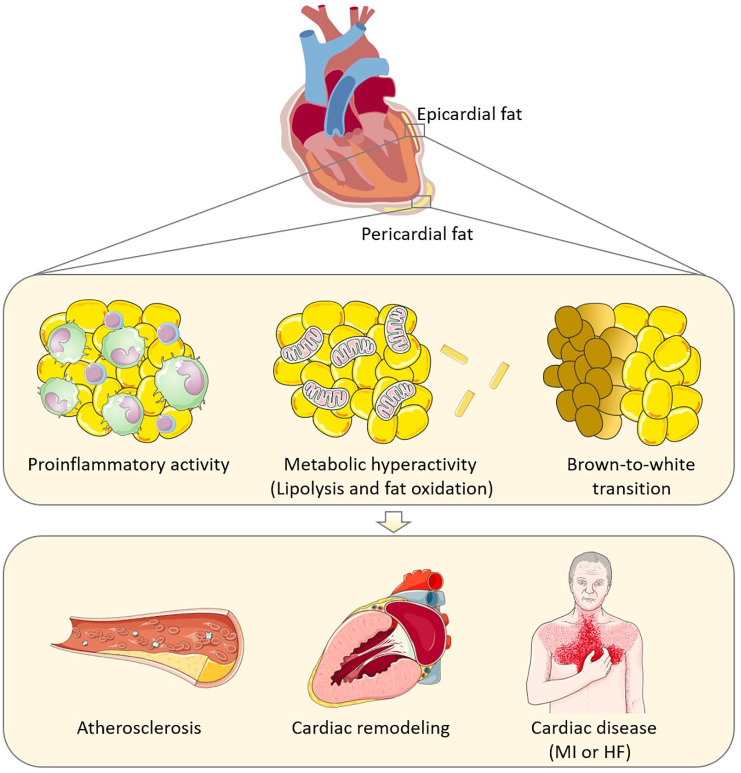
Later observational studies showed that the amount of EAT is increased in patients with T2D and CVD,25 and associated with intramyocardial fat accumulation.43–46 Translational mechanistic studies have shown that factors secreted from EAT disrupt fatty acid beta oxidation in cardiomyocytes, which normally is their major source of energy, accounting for 60–70% of the ATP produced.47 Thus, EAT is now recognized as a metabolic tissue, having the highest rates of lipolysis among the VAT depots, which, in obesity and T2D, may accelerate atherosclerosis in the coronary vasculature and lipotoxicity of the cardiomyocyte. Specifically for patients with T2D, it was found that the fatty acid profile of EAT was different from that of patients without T2D, and there was a decrease in 16:0 and omega 3 fatty acids and an increase of trans and conjugated fatty acids, which may worsen the formation of atheroma in the neighboring arteries.48 Secretory products from EAT from patients with T2D have also been shown to impair cardiomyocyte contractile function and fat oxidation.49 Overall, this metabolic hyperactivity indicates that EAT has a pathophysiological potential that may be aggravated by diabetes (Figure 1).
Figure 1.
Mechanisms whereby epicardial (and potentially also pericardial) adipose tissue expansion in T2D may accelerate atherosclerosis, myocardial remodeling and diastolic dysfunction, MI, or HF. Clipart provided by Servier Medical Art.50
HF, heart failure; MI, myocardial infarction; T2D, type 2 diabetes.
Adipokine secretion of EAT
The recognition of EAT as an active secretory tissue came from a seminal finding by Mazurek and collegues in 2004.28 They showed that, in patients with coronary artery disease (CAD), EAT has a higher expression of pro-inflammatory cytokines (TNFα, IL-6, IL-1β, and others), a higher infiltration of chronic inflammatory cells, and secretes more pro-inflammatory cytokines compared with subcutaneous fat (SAT) biopsies from the same patient.28 Moreover, adiponectin expression was found to be lower in EAT from patients with CAD compared with non-CAD patients,51 and was lower in EAT compared with SAT.52 Chatterjee et al. expanded the adipokine list by demonstrating that cultured EAT adipocytes secrete IL-8.53 Subsequently, we found an indication that the local inflammatory response identified from the above studies could be measured systemically since EAT was associated with increased levels of IL-8 in plasma.31 However, whether EAT contributes markedly to the systemic low-grade inflammation needs to be investigated. Since then, others have confirmed the pro-inflammatory transcriptome of EAT,54–58 and indicated that EAT is more inflamed compared with intra-abdominal VAT.55 In patients with CAD, the secretome of EAT compared with SAT (in conditioned media from tissue explants) showed an atherogenic and inflammatory protein secretion profile.59 Moreover, a few studies have shown that the secretome of EAT disrupts cardiomyocyte metabolism,47 depresses cardiomyocyte contractile function,49 and alters expression of adhesion markers of primary cardiac endothelial cells.60 A recent intervention study in a rat model of myocardial infarction (MI) showed that surgical removal of EAT improves myocardial function following MI.61 Causal evidence was further provided in a pig model of atherosclerosis, where resection of EAT from the anterior descending coronary artery reduced atherosclerotic plaque progression exclusively at the site of adipectomy.62 Obese mice fed a high fat diet specifically induced a pro-inflammatory adipokine state and increased adipocyte size in pericardial fat.63 Specific for T2D, a study by Sacks et al. indicated a predominantly pro-inflammatory adipokine signature in EAT from patients with metabolic syndrome and T2D,64 and this was confirmed by another research group who demonstrated that adiponectin gene expression was reduced, whereas CD68, MCP-1, and adipocyte size were increased in EAT from patients with T2D versus controls.65 The immune cell population found in EAT may also be influenced by diabetes since dendritic cells (professional antigen-presenting cells contributing to regulation of lymphocyte immune response) were downregulated,66 whereas infiltrating pro-inflammatory macrophages were upregulated in EAT from patients with T2D. 67 Thus, EAT in T2D is particularly inflamed, which could accelerate atherosclerosis and cardiac complications in this population (Figure 1).
Thermogenic capacity of EAT
EAT is hypothesized to offer cardiac cryoprotection due to its thermogenic capacity, resembling that of brown/beige adipocytes.60,68,69 However, it is not known whether the thermogenic properties are functional in adult humans. Moreover, the heat generated by EAT thermogenesis may be of little or no physiological significance compared with the heat generated by the cardiomyocyte during the contractile cycle. Interestingly, a brown-to-white transition of EAT, due to downregulation of brown adipose tissue and upregulation of white adipose tissue associated genes, has been suggested to occur in patients with CAD compared with non-CAD.70 While it is not known whether T2D induces brown-to-white transition in EAT, or if this plays a role in mediating the cardiometabolic disease progression,71 a study by Moreno-Santos et al. supports this idea by showing that T2D was associated with decreased expression of PGC1α and UCP1 mRNA in EAT of patients with T2D and CAD, likely reflecting a loss of brown-like fat features72 (Figure 1).
PAT pathophysiology
Human PAT is located within, and on the external site of, the pericardium and is of a different origin (primitive thoracic mesenchyme) than EAT (splanchnopleuric mesoderm).30,73–75 PAT is supplied by blood from the thoracic vasculature and it is not in direct contact with the myocardium.30,73,74 Therefore, cardiac physiology may only be affected indirectly by PAT and it is also not directly affected by the “inside-to-out” paracrine signaling of the myocardium to the adipose tissue.21,36 Despite these marked differences of the depots, EAT and PAT have similar transcriptional profiles,76 and, when EAT and PAT are combined, this entire fat pad remains associated with an increased risk of future CVD, which has been shown in prospective studies.31,77–82 Similar to EAT, PAT has a higher expression of pro-inflammatory adipokines compared with intraabdominal VAT.63,83 While one paper suggests PAT to be more closely associated with cardiovascular risk factors compared with EAT,84 others have found that PAT alone does not predict future CVD and all-cause mortality in patients with diabetes.31 Overall, the literature points towards a role of PAT in cardiac disease pathology, but the physiology of PAT and its importance in cardiac disease progression in patients with T2D is not fully clarified.
Altogether, these mechanistic in vitro and in vivo studies indicate a pro-inflammatory, proatherogenic, and cardiotoxic effect of EAT and PAT in general and in diabetes. However, an important limitation is that, in all studies, the fat is obtained from patients/animals with established CVD undergoing open heart surgery, which per se affects the physiology of EAT and PAT and limits the conclusions. In the next section, we move from mechanistic to epidemiological and clinical studies investigating whether EAT plays a particularly important role for CVD progression in T2D.
High levels of EAT in patients with T2D
Several studies have reported that patients with T2D have higher levels of EAT compared with non-diabetic controls (Table 1). In 2009, Wang and colleagues showed that mean EAT was 166.1 ± 60.6 cm3 in patients with T2D compared with 123.4 ± 41.8 cm3 in patients without diabetes.85 This finding of high amounts of EAT in patients with T2D was confirmed by several subsequent studies,86–88 including a recent large cohort of 1000 patients.32 In 2014, a meta-analysis confirmed the association of EAT and parameters of the metabolic syndrome,89 and, in 2019, a meta-analysis including 13 studies confirmed the association of EAT and T2DM.25 While it is now clear that EAT is increased in patients with T2D, it is not established in type 1 diabetes (T1D). Although some studies do support a role of EAT in cardiac disease in T1D,90–92 it has recently been reported that EAT volume was not higher and not associated with coronary atherosclerosis in T1D patients.93 In support of this, we recently found patients with T1D to have lower cardiac adipose tissue volumes compared with patients with T2D, and levels similar to those of controls.94
Table 1.
Amount of EAT in patients with and without diabetes.
| Study | DM | Epicardial adipose tissue |
Number (T2D versus controls) |
Measurement tool | Year published | |
|---|---|---|---|---|---|---|
| T2D | Controls | |||||
| Iacobellis et al.90 | T1 | 7.2 ± 2.1 mm | 4.9 ± 2.5 mm | 30 (15 versus 15) | Echocardiography | 2014 |
| Chambers et al.91 | T1 | 1.65 ± 0.44 mm | 1.37 ± 0.27 mm | 40 (20 versus 20) | Echocardiography | 2019 |
| Cetin et al.88 | T2 | 6.0 ± 1.5 mm | 4.42 ± 1.0 mm | 139 (99 versus 40) | Echocardiography | 2013 |
| Kang et al.95 | T2 | 5.4 (4.2, 7.4) mm | 3.9 (2.9, 4.8) mm | 321 (40 versus 281) | Echocardiography | 2018 |
| Christensen et al.32 | T2 | 4.6 ± 1.8 mm | 3.4 ± 1.2 mm | 1004 (770 versus 234) | Echocardiography | 2019 |
| Ojeda-Peña et al.96 | T2 | 7.0 mm¤ | 5.7 mm¤ | 60 (30 versus 30) | Echocardiography | 2016 |
| Vasques et al.97 | T2 | 10.2 ± 2.8 mm | 8.2 ± 1.8 mm | 49 (31 versus 18) | Echocardiography | 2015 |
| Peraza-Zaldivar et al.98 | T2 | 8 (7, 9) mm# | 6 (2, 10) mm# | 40 (22 versus 18) | Echocardiography | 2016 |
| Seker et al.99 | T2 | 6.5 ± 0.7 mm | 5.3 ± 1.0 mm | 454 (186 versus 268) | Echocardiography | 2017 |
| Chun et al.100 | T2 | 17.6 ± 6.7 mm | 14.4 ± 5.9 mm | 1048 (141 versus 907) | CT | 2015 |
| Wang et al.101 | T2 | 5.0 ± 1.2 mm | 3.1 ± 0.8 mm | 100 (68 versus 32) | Echocardiography | 2017 |
| Yazici et al.102 | T1 | 3.3 ± 1.1 mm | 2.3 ± 0.3 mm | 79 (36 versus 43) | Echocardiography | 2011 |
| Tonbul et al.86 | n/a | 215.5 (126.5, 271.2) cm3 | 116.0 (91.6–139.4) cm3 | 60 (17 versus 43) | CT | 2011 |
| Versteylen et al.103 | T2 | 98 ± 41 cm3 | 75 ± 34 cm3 | 292 (83 versus 209) | CT | 2012 |
| Wang et al.85 | T2 | 166.1 ± 60.6 cm3 | 123.4 ± 41.8 cm3 | 127 (49 versus 78) | CT | 2009 |
| Milanese et al.104 | T2 | 112.9 [21.4, 442.2] ml | 82.6 [11.3, 318] ml | 596 (215 versus 381) | CT | 2019 |
| Svanteson et al. 93 | T1 | 52.3 (36.1–65.5) cm3 | 55 (38.3–79.6) cm3a | 148 (88 versus 60) | CT | 2019 |
| Zobel et al.105 | T1 | 106 ± 78 ml | 99 ± 61 mla,b | 90 (60 versus 30) | CT | 2020 |
| Zobel et al. 105 | T2 | 228 ± 97 ml | 99 ± 61 mlb | 90 (60 versus 30) | CT | 2020 |
| Yang et al.82 | T2 | 89 ± 24.6 ml | 67.6 ± 26.7 ml | 407 (50 versus 357) | CT | 2013 |
| Akyürek et al.106 | T2 | 172.8 ± 64.9 cm3 | 68.9 ± 37.7 cm3 | 152 (90 versus 62) | CT | 2015 |
| Gaborit et al.87 | T2 | 213 ± 34 ml | 141 ± 18 ml | 30 (13 versus 17) | MR | 2012 |
| Rado et al.107 | T2 | 7.7 (5, 10) cm3# | 10.3 (7, 14) cm3#x | 272 (52 versus 220) | MR | 2019 |
| van Woerden et al.108 | T2 | 116 ± 10 ml/m# | 100 ± 10 ml/m# | 64 (28 versus 36) | MR | 2018 |
| Gullaksen et al.109 | T2 | 119 ± 49 mm3 | 86 ± 40 mm3 | 103 (44 versus 59) | CT | 2019 |
IQR [range].
No SD or IQR available.
Estimated or partly estimated from a figure.
Not significant.
No discrimination between EAT and PAT.
CT, computed tomography; EAT, epicardial adipose tissue; IQR, interquartile range; MR, magnetic resonance; PAT, pericardial adipose tissue; SD, standard deviation; T2D, type 2 diabetes; T1D, type I diabetes.
Taken together, while not yet established in T1D, EAT is increased in patients with T2D, suggesting a potential importance in CVD progression in this population, which will be discussed below.
Does EAT drive the association of T2D and CVD?
While several large-scale epidemiological studies,79,80,110–116 including recent meta-analyses,117,118 have implicated a role of EAT in provoking atherosclerosis independently of diabetes, the increased level of EAT in T2D may suggest it is an important link coupling diabetes and cardiovascular disease (Table 2). Wang and colleagues were among the first to describe an association of EAT volume with coronary artery calcium (CAC) scores and significant coronary lesions (more than 50% stenosis) in asymptomatic patients with T2D.85 Others have reported similar findings,31,119 including Kim et al., who found an association with coronary lesions, but, on the contrary, reported that EAT was not independently associated with silent myocardial ischemia based on first-pass myocardial perfusion magnetic resonance (MR) images acquired during adenosine stress and at rest.120 Other cross-sectional studies have also reported that EAT in T2D is not associated with myocardial perfusion or microvascular dysfunction, which raise uncertainty of the functional importance of EAT in T2D.105,121 Nevertheless, an early prospective study by Yerramasu et al. found that EAT volume was an independent marker for the presence and severity of coronary calcium burden in 333 asymptomatic patients with T2D without prior history of CVD, and was associated with progression of CAC, whereas traditional measures of obesity were not independently associated with these endpoints.122 Other prospective studies have emerged since then, including a study by our group performed in a cohort of 200 patients with T2D.31 In this latter study, high cardiac adipose tissue levels (EAT+PAT) were independently associated with increased risk of incident CVD or all-cause mortality after 6.1 years of follow up. We confirmed this finding in a larger prospective study of 1030 patients with T2D, where the results additionally indicated a gender-specific role of EAT as its predictive potential for CVD was increased for men compared with women after 4.7 years of follow up.33 We also found that EAT modestly improved risk prediction when added to a model including traditional CVD risk parameters.
Table 2.
Association of EAT and CVD in T2D.
| Study | Association of EAT with | Design | Year |
|---|---|---|---|
| Wang et al.85 | CAC score, coronary lesions | Cross sectional | 2009 |
| Kazlauskaite et al.123 | Diastolic dysfunction | Cross sectional | 2010 |
| Yerramasu et al.122 | CAC score, CAC progression | Prospective | 2012 |
| Versteylen et al.103 | Coronary artery disease | Cross sectional | 2012 |
| Kim et al.120 | Significant coronary stenosis, myocardial ischemia | Cross sectional | 2012 |
| Chen et al.121 | Myocardial microvascular dysfunctiona | Cross sectional | 2014 |
| Levelt et al.124 | Cardiac contractile dysfunction (impaired systolic and diastolic strain rates) | Cross sectional | 2016 |
| Uygur et al.125 | Coronary atherosclerosis | Cross sectional | 2017 |
| Christensen et al.31 | CAC score, incident cardiovascular events and all-cause mortalityb | Prospective | 2017 |
| Reinhard et al.119 | CAC score | Cross sectional | 2019 |
| Christensen et al.32 | Reduced diastolic function | Cross sectional | 2019 |
| Christensen et al.33 | Incident cardiovascular events and all-cause mortality | Prospective | 2019 |
CAC, coronary artery calcium; CVD, cardiovascular disease; EAT, epicardial adipose tissue; PAT, pericardial adipose tissue; T2D, type 2 diabetes.
Not significant.
Total cardiac fat (EAT+PAT).
Overall, whereas the main body of evidence suggests a role for EAT in the development of CVD in T2D, EAT is a heterogenous fat depot and may have different atherogenic potential depending on its location. Uygur et al. have suggested that the left atrioventricular groove EAT volume was superior in the prediction of CAD in patients with T2D without CAD history,125 and Maimaituxun et al. identified that the local fat thickness surrounding the left anterior descending artery (LAD), when compared with EAT at other locations, was a useful surrogate marker for estimating the presence, severity, and extent of CAD, independent of classical cardiovascular risk factors.126 A post hoc analysis from the CRISP CT study identified that the perivascular (epicardial) fat attenuation index (which captures coronary inflammatory load) at both LAD and the right coronary artery were predictive of all-cause and cardiac mortality and improved risk prediction algorithms in a mixed population of patients with and without T2D.127 This finding suggests that the physiological state of EAT or PAT (e.g. inflammatory or brown-fat activity) compared with the amount may be a better estimate for the risk of CVD.
Regarding cardiac function, several studies have shown that EAT is associated with diastolic dysfunction.88,128–134 Levelt et al. showed that lean versus obese patients with T2D have lower degree of EAT and better cardiac function,124 indicating that the adipose load including EAT is a factor in mediating cardiac dysfunction and, in particular, mediating derangements in left ventricle (LV) mass and volume.77 EAT has also been associated with diastolic dysfunction in patients with newly diagnosed T2D,123 as well as with longer diabetes duration.32 A few studies also indicate a role for cardiac fat in cardiac systolic dysfunction, both in general,108 and in patients with T2D specifically.32
Taken together, there is considerable evidence to suggest that EAT is associated with an increased risk of CVD in general, and in patients with T2D in particular. EAT is also associated with reduced diastolic function in general and in T2D, and, although only few studies exist, there is emerging evidence of a role for EAT in systolic heart failure (Figure 1). Despite the clear evidence of EAT being a biomarker of heart disease and CVD, the question of whether T2D aggravates the pathogenic potential of EAT remains controversial.
Clinical potential of EAT in cardiovascular risk prediction
From both mechanistic and epidemiological studies, it is clear that EAT is associated with increased cardiovascular risk, and some studies suggest that it may also have potential to guide clinical decision making.33,127,135 However, several aspects need clarification before the clinical relevance of EAT can be fully determined (Figure 2).
Figure 2.
Future questions for epicardial and pericardial adipose tissue research.
CT, computed tomography; CVD, cardiovascular disease; EAT, epicardial adipose tissue; MRI, magnetic resonance imaging; PAT, pericardial adipose tissue.
EAT can be measured by echocardiography, CT, or cardiac magnetic resonance imaging (MRI).136 The measurement of EAT by echocardiography has several limitations, namely the discrimination of EAT and PAT can be difficult,3 EAT can be misinterpreted as pericardial effusion,2 and the restricted acoustic window can impair a valid reflection of the total fat volume and fail to identify regional differences in fat distribution. Therefore, echocardiography exclusively allows for a rough two-dimensional estimation of the adipose tissue beds.1,5 Conversely, CT and cardiac MRI are gold standards and allow for volumetric quantifications of EAT.136 However, quantification in clinical practice, even by the gold standard, is challenging because of lack of sensitivity and specificity, and because it is technically difficult and there is a possibility for high noise and confounding due to, for example, interference of heart beats, water content, and fat droplets from parenchymal cells during image acquisition.137,138 Thus, a uniform standardized method for EAT quantification has not yet been determined, which has prevented the establishment of threshold values for physiological and pathological levels of EAT.
Another major challenge in the evaluation of EAT as a novel cardiac risk factor is the physiologic similarities between fat depots, which makes quantifying their independent contributions to cardiac risk difficult.77,125 Whereas some causal evidence of an independent role of EAT in the development of CVD has been obtained in animal studies,62 it is generally lacking in humans due to the difficulty in specifically targeting the EAT. Despite a previous study by our group that indicated cardiac fat was associated with CVD and all-cause mortality independently of BMI,31 it remains to be fully clarified whether EAT performs better than traditional anthropometric risk markers (e.g., BMI or waist circumference) or other visceral fat depots in predicting CVD risk.
For EAT to be a clinically important risk factor, we need to understand how the depot can be modified. Emerging evidence shows that EAT can be reduced by pharmacological therapies including GLP-1 analogues and SLGT2 inhibitors.139–143 It is, however, not known whether the cardioprotective effects of these drugs are mediated through the reductions in EAT. Whether EAT can also be targeted by lifestyle modifications for example, exercise, has been controversial,144 but recent studies support this idea.145,146 A recent study by our group suggests that exercise training reduces both EAT and PAT, without a change in total fat mass, indicating that exercise training may be a means to specifically target these fat depots.147 We also identified that the mechanism by which exercise targets EAT is through an IL-6 dependent mechanism, since blocking of the IL-6 receptor by Tocilizumab (a human monoclonal antibody) abolished exercise-induced EAT reductions.148 This disclosure of one of the mechanisms regulating EAT is important in order to find potential novel treatment targets. In general, both mechanistic and large longitudinal studies and properly designed intervention studies are needed to identify ways to specifically target EAT.
Overall, we now know from several observational studies that EAT shows promise as a modifiable cardiac risk factor. The underlying mechanisms by which EAT may accelerate atherosclerosis and myocardial damage have also been investigated in several studies and summarized in excellent recent reviews by Packer,26,27,149 Iacobellis34,150 and others,21,29,128,151 who shaped the idea that EAT plays a critical role as a metabolic transducer of systematic inflammation and thereby exerts deleterious effects on the myocardium and coronary arteries. Despite this, there are several aspects to be clarified before we understand whether EAT is a clinically relevant risk factor that will improve risk stratification and guide future clinical decision-making. Some essential aspects will be to establish how, and at what location, this depot should be measured, whether we need to measure the total amount of fat (EAT + PAT) or rather the physiological state (e.g. inflammatory or brown fat activity), and whether EAT can be used in both males and females, and in the general population or only in sub-populations, for example, high-risk patients with T2D. We also need to understand how, and to what degree, EAT should be targeted to translate into clinically relevant reductions in cardiovascular risk (Figure 2).
Conclusion
EAT and PAT are emerging as potential clinically relevant cardiovascular risk markers, but several unanswered questions remain about these regional depots. The next leap forward will be to clearly establish the clinical relevance of EAT and PAT and their relative contributions to CVD and the predictive potential, both in the general population and in patients with T2D. Subsequently modifying EAT and PAT may become targets to reduce the excess cardiovascular morbidity and mortality in diabetes and obesity.
Footnotes
Author Contribution(s): Regitse Højgaard Christensen: Conceptualization; Formal analysis; Investigation; Methodology; Visualization; Writing-original Draft; Writing-review & editing.
Bernt Johan von Scholten: Conceptualization; Supervision; Writing-review & editing.
Louise Lang Lehrskov: Conceptualization; Writing-review & editing.
Peter Rossing: Conceptualization; Writing-review & editing.
Peter Godsk Jørgensen: Conceptualization; Investigation; Supervision; Writing-review & editing.
Conflict of interest statement: The authors declare that there is no conflict of interest associated with this manuscript. PR has received consultancy and/or speaking fees (to his institution) from Astellas, AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Merck, Novo Nordisk, Sanofi Aventis, and Vifor.
Funding: Research grants from AbbVie, AstraZeneca and Novo Nordisk. BJvS is now employed at Novo Nordisk and has equity interest in Novo Nordisk. PGJ reports having received lecture fees from Novo Nordisk.
ORCID iDs: Regitse Højgaard Christensen  https://orcid.org/0000-0001-5316-5341
https://orcid.org/0000-0001-5316-5341
Peter Godsk Jørgensen  https://orcid.org/0000-0002-1217-8944
https://orcid.org/0000-0002-1217-8944
Contributor Information
Regitse Højgaard Christensen, Center for Inflammation and Metabolism/Center for Physical Activity Research, Dept. 7641, Rigshospitalet, Blegdamsvej 9, Kbh Ø, 2100, Denmark; Steno Diabetes Center Copenhagen, Gentofte, Denmark.
Bernt Johan von Scholten, Steno Diabetes Center Copenhagen, Gentofte, Denmark.
Louise Lang Lehrskov, Center for Inflammation and Metabolism/Center for Physical Activity Research, Rigshospitalet, Denmark.
Peter Rossing, Steno Diabetes Center Copenhagen, Gentofte, Denmark; Department of Clinical Medicine, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark.
Peter Godsk Jørgensen, Department of Cardiology, Herlev-Gentofte Hospital, Denmark.
References
- 1. Saeedi P, Petersohn I, Salpea P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the international diabetes federation diabetes Atlas, 9th edition Diabetes Res Clin Pract 2019; 157: 107843. [DOI] [PubMed] [Google Scholar]
- 2. Fox CS, Coady S, Sorlie PD, et al. Trends in cardiovascular complications of diabetes. JAMA 2004; 29: 2495–2499. [DOI] [PubMed] [Google Scholar]
- 3. Barr ELM, Zimmet PZ, Welborn TA, et al. Risk of cardiovascular and all-cause mortality in individuals with diabetes mellitus, impaired fasting glucose, and impaired glucose tolerance: the Australian diabetes, obesity, and lifestyle study (AusDiab). Circulation 2007; 116: 151–157. [DOI] [PubMed] [Google Scholar]
- 4. Kannel WB, McGee DL. Diabetes and cardiovascular disease: the Framingham study. JAMA 1979; 241: 2035–2038. [DOI] [PubMed] [Google Scholar]
- 5. Kannel WB, McGee DL. Diabetes and glucose tolerance as risk factors for cardiovascular disease: the Framingham study. Diabetes Care 1979; 2: 120–126. [DOI] [PubMed] [Google Scholar]
- 6. Kannel WB, Hjortland M, Castelli WP. Role of diabetes in congestive heart failure: the Framingham study. Am J Cardiol 1974; 34: 29–34. [DOI] [PubMed] [Google Scholar]
- 7. Saeedi P, Salpea P, Karuranga S, et al. Mortality attributable to diabetes in 20–79 years old adults, 2019 estimates: results from the international diabetes federation diabetes Atlas, 9th edition Diabetes Res Clin Pract. Epub ahead of print 15 February 2020. DOI: 10.1016/j.diabres.2020.108086. [DOI] [PubMed] [Google Scholar]
- 8. Cavender MA, Steg PG, Smith SC, Jr, et al. Impact of diabetes mellitus on hospitalization for heart failure, cardiovascular events, and death: outcomes at 4 years from the reduction of atherothrombosis for continued health (REACH) registry. Circulation 2015; 132: 923–931. [DOI] [PubMed] [Google Scholar]
- 9. Chen HF, Ho CA, Li CY. Risk of heart failure in a population with type 2 diabetes versus a population without diabetes with and without coronary heart disease. Diabetes Obes Metab 2019; 21: 112–119. [DOI] [PubMed] [Google Scholar]
- 10. Franco OH, Steyerberg EW, Hu FB, et al. Associations of diabetes mellitus with total life expectancy and life expectancy with and without cardiovascular disease. Arch Intern Med 2007; 167: 1145–1151. [DOI] [PubMed] [Google Scholar]
- 11. Preis SR, Hwang SJ, Coady S, et al. Trends in all-cause and cardiovascular disease mortality among women and men with and without diabetes mellitus in the Framingham heart study, 1950 to 2005. Circulation 2009; 119: 1728–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Boudina S, Abel ED. Diabetic cardiomyopathy, causes and effects. Rev Endocr Metab Disord 2010; 11: 31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bussey CT, de Leeuw AE, Lamberts RR. Increased haemodynamic adrenergic load with isoflurane anaesthesia in type 2 diabetic and obese rats in vivo. Cardiovasc Diabetol 2014; 13: 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. DeFronzo RA. Insulin resistance, lipotoxicity, type 2 diabetes and atherosclerosis: the missing links. The Claude Bernard lecture 2009. Diabetologia 2010; 53: 1270–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Neeland IJ, Ross R, Després JP, et al. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: a position statement. Lancet Diabetes Endocrinol 2019; 7: 715–725. [DOI] [PubMed] [Google Scholar]
- 16. Després JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature 2006; 444: 881–887. [DOI] [PubMed] [Google Scholar]
- 17. Després JP, Moorjani S, Lupien PJ, et al. Regional distribution of body fat, plasma lipoproteins, and cardiovascular disease. Atherosclerosis 1990; 10: 497–511. [DOI] [PubMed] [Google Scholar]
- 18. Kim SH, Després JP, Koh KK. Obesity and cardiovascular disease: friend or foe? Eur Heart J 2016; 37: 3560–3568. [DOI] [PubMed] [Google Scholar]
- 19. Wende AR, Abel ED. Lipotoxicity in the heart. Biochim Biophys Acta 2010; 1801: 311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tchkonia T, Thomou T, Zhu Y, et al. Mechanisms and metabolic implications of regional differences among fat depots. Cell Metab 2013; 17: 644–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Oikonomou EK, Antoniades C. The role of adipose tissue in cardiovascular health and disease. Nat Rev Cardiol 2019; 16:83–99. [DOI] [PubMed] [Google Scholar]
- 22. Ibrahim MM. Subcutaneous and visceral adipose tissue: structural and functional differences. Obes Rev 2010; 11: 11–18. [DOI] [PubMed] [Google Scholar]
- 23. Vague J. The degree of masculine differentiation of obesities: a factor determining predisposition to diabetes, atherosclerosis, gout, and uric calculous disease. Am J Clin Nutr 1956; 4: 20–34. [DOI] [PubMed] [Google Scholar]
- 24. Arner P. Human fat cell lipolysis : biochemistry, regulation and clinical role. Best Pract Res Clin Endocrinol Metab 2005; 19: 471–482. [DOI] [PubMed] [Google Scholar]
- 25. Li Y, Liu B, Li Y, et al. Epicardial fat tissue in patients with diabetes mellitus: a systematic review and meta-analysis. Cardiovasc Diabetol 2019; 18: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Packer M. Critical role of the epicardium in mediating cardiac inflammation and fibrosis in patients with type 2 diabetes. Diabetes Obes Metab 2019; 21: 1765–1768. [DOI] [PubMed] [Google Scholar]
- 27. Packer M. Epicardial adipose tissue may mediate deleterious effects of obesity and inflammation on the myocardium. J Am Coll Cardiol 2018; 71: 2360–2372. [DOI] [PubMed] [Google Scholar]
- 28. Mazurek T, Zhang L, Zalewski A, et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation 2003; 108: 2460–2466. [DOI] [PubMed] [Google Scholar]
- 29. Mazurek T, Opolski G. Pericoronary adipose tissue: a novel therapeutic target in obesity-related coronary atherosclerosis. J Am Coll Nutr 2015; 34: 244–254. [DOI] [PubMed] [Google Scholar]
- 30. Noyes AM, Dua K, Devadoss R, et al. Cardiac adipose tissue and its relationship to diabetes mellitus and cardiovascular disease. World J Diabetes 2014; 5: 868–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Christensen RH, von Scholten BJ, Hansen CS, et al. Epicardial, pericardial and total cardiac fat and cardiovascular disease in type 2 diabetic patients with elevated urinary albumin excretion rate. Eur J Prev Cardiol 2017; 24: 1517–1524. [DOI] [PubMed] [Google Scholar]
- 32. Christensen RH, Hansen CS, von Scholten BJ, et al. Epicardial and pericardial adipose tissues are associated with reduced diastolic and systolic function in type 2 diabetes. Diabetes Obes Metab 2019; 21: 2006–2011. [DOI] [PubMed] [Google Scholar]
- 33. Christensen RH, Scholten BJ, Von, Hansen CS, et al. Epicardial adipose tissue predicts incident cardiovascular disease and mortality in patients with type 2 diabetes. Cardiovasc Diabetol 2019; 18: 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Iacobellis G. Local and systemic effects of the multifaceted epicardial adipose tissue depot. Nat Rev Endocrinol 2015; 11: 363–371. [DOI] [PubMed] [Google Scholar]
- 35. Iacobellis G, Corradi D, Sharma AM. Epicardial adipose tissue: anatomic, biomolecular and clinical relationships with the heart. Nat Clin Pract Cardiovasc Med 2005; 2: 536–543. [DOI] [PubMed] [Google Scholar]
- 36. Antonopoulos AS, Antoniades C. The role of epicardial adipose tissue in cardiac biology: classic concepts and emerging roles. J Physiol 2017; 595: 3907–3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cherian S, Lopaschuk GD, Carvalho E. Cellular cross-talk between epicardial adipose tissue and myocardium in relation to the pathogenesis of cardiovascular disease. Am J Physiol Endocrinol Metab 2012; 303: e937–e949. [DOI] [PubMed] [Google Scholar]
- 38. Schindler TH. Epicardial adipose tissue: a new cardiovascular risk marker? Int J Cardiol 2019; 278: 263–264. [DOI] [PubMed] [Google Scholar]
- 39. Yudkin JS, Eringa E, Stehouwer CDA. “Vasocrine” signalling from perivascular fat: a mechanism linking insulin resistance to vascular disease. Lancet 2005; 365: 1817–1820. [DOI] [PubMed] [Google Scholar]
- 40. Iacobellis G, Malavazos AE, Corsi MM. Epicardial fat: from the biomolecular aspects to the clinical practice. Int J Biochem Cell Biol 2011; 43: 1651–1654. [DOI] [PubMed] [Google Scholar]
- 41. Marchington JM, Mattacks CA, Pond CM. Adipose tissue in the mammalian heart and pericardium: structure, foetal development and biochemical properties. Comp Biochem Physiol B 1989; 94: 225–232. [DOI] [PubMed] [Google Scholar]
- 42. Smith HL, Willius FA. Adiposity of the heart: a clinical and pathologic study of one hundred and thirty-six obese patients. Arch Intern Med 1933; 52: 911–931. [Google Scholar]
- 43. Ng ACT, Strudwick M, van der Geest RJ, et al. Impact of epicardial adipose tissue, left ventricular myocardial fat content, and interstitial fibrosis on myocardial contractile function. Circ Cardiovasc Imaging 2018; 11: e007372. [DOI] [PubMed] [Google Scholar]
- 44. Kankaanpää M, Lehto HR, Pärkkä JP, et al. Myocardial triglyceride content and epicardial fat mass in human obesity: relationship to left ventricular function and serum free fatty acid levels. J Clin Endocrinol Metab 2006; 91: 4689–4695. [DOI] [PubMed] [Google Scholar]
- 45. Malavazos AE, Di Leo G, Secchi F, et al. Relation of echocardiographic epicardial fat thickness and myocardial fat. Am J Cardiol 2010; 105: 1831–1835. [DOI] [PubMed] [Google Scholar]
- 46. Antonopoulos AS, Antoniades C. Cardiac magnetic resonance imaging of epicardial and intramyocardial adiposity as an early sign of myocardial disease. Circ Cardiovasc Imaging 2018; 11: e008083. [DOI] [PubMed] [Google Scholar]
- 47. Blumensatt M, Fahlbusch P, Hilgers R, et al. Secretory products from epicardial adipose tissue from patients with type 2 diabetes impair mitochondrial β-oxidation in cardiomyocytes via activation of the cardiac renin–angiotensin system and induction of miR-208a. Basic Res Cardiol 2017; 112: 2. [DOI] [PubMed] [Google Scholar]
- 48. Pezeshkian M, Mahtabipour MR. Epicardial and subcutaneous adipose tissue fatty acids profiles in diabetic and non-diabetic patients candidate for coronary artery bypass graft. Bioimpacts 2013; 3: 83–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Greulich S, Maxhera B, Vandenplas G, et al. Secretory products from epicardial adipose tissue of patients with type 2 diabetes mellitus induce cardiomyocyte dysfunction. Circulation 2012; 126: 2324–2334. [DOI] [PubMed] [Google Scholar]
- 50. Les Laboratories Servier. Servier medical art [Internet], https://smart.servier.com/ (accessed 1 February 2020).
- 51. Iacobellis G, Pistilli D, Gucciardo M, et al. Adiponectin expression in human epicardial adipose tissue in vivo is lower in patients with coronary artery disease. Cytokine 2005; 29: 251–255. [DOI] [PubMed] [Google Scholar]
- 52. Bambace C, Telesca M, Zoico E, et al. Adiponectin gene expression and adipocyte diameter: a comparison between epicardial and subcutaneous adipose tissue in men. Cardiovasc Pathol 2011; 20: e153–e156. [DOI] [PubMed] [Google Scholar]
- 53. Chatterjee TK, Stoll LL, Denning GM, et al. Proinflammatory phenotype of perivascular adipocytes: influence of high-fat feeding. Circ Res 2009; 104: 541–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sacks HS, Fain JN, Cheema P, et al. Depot-specific overexpression of proinflammatory, redox, endothelial cell, and angiogenic genes in epicardial fat adjacent to severe stable coronary atherosclerosis. Metab Syndr Relat Disord 2011; 9: 433–439. [DOI] [PubMed] [Google Scholar]
- 55. Imoto-Tsubakimoto H, Takahashi T, Ueyama T, et al. Serglycin is a novel adipocytokine highly expressed in epicardial adipose tissue. Biochem Biophys Res Commun 2013; 432: 105–110. [DOI] [PubMed] [Google Scholar]
- 56. McAninch EA, Fonseca TL, Poggioli R, et al. Epicardial adipose tissue has a unique transcriptome modified in severe coronary artery disease. Obesity (Silver Spring) 2015; 23: 1267–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Camarena V, Sant D, Mohseni M, et al. Novel atherogenic pathways from the differential transcriptome analysis of diabetic epicardial adipose tissue. Nutr Metab Cardiovasc Dis 2017; 27: 739–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Gaborit B, Venteclef N, Ancel P, et al. Human epicardial adipose tissue has a specific transcriptomic signature depending on its anatomical peri-atrial, peri-ventricular, or peri-coronary location. Cardiovasc Res 2015; 108: 62–73. [DOI] [PubMed] [Google Scholar]
- 59. Salgado-Somoza A, Teijeira-Fernández E, Fernández ÁL, et al. Changes in lipid transport-involved proteins of epicardial adipose tissue associated with coronary artery disease. Atherosclerosis 2012; 224: 492–499. [DOI] [PubMed] [Google Scholar]
- 60. Chechi K, Voisine P, Mathieu P, et al. Functional characterization of the Ucp1-associated oxidative phenotype of human epicardial adipose tissue. Sci Rep 2017; 7: 15566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Chang HX, Zhao XJ, Zhu QL, et al. Removal of epicardial adipose tissue after myocardial infarction improves cardiac function. Herz 2018; 43: 258–264. [DOI] [PubMed] [Google Scholar]
- 62. McKenney ML, Schultz KA, Boyd JH, et al. Epicardial adipose excision slows the progression of porcine coronary atherosclerosis. J Cardiothorac Surg 2014; 9: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wang CY, Li SJ, Wu TW, et al. The role of pericardial adipose tissue in the heart of obese minipigs. Eur J Clin Invest 2018; 48: e12942. [DOI] [PubMed] [Google Scholar]
- 64. Sacks HS, Fain JN, Cheema P, et al. Inflammatory genes in epicardial fat contiguous with coronary atherosclerosis in the metabolic syndrome and type 2 diabetes: changes associated with pioglitazone. Diabetes Care 2011; 34: 730–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Bambace C, Sepe A, Zoico E, et al. Inflammatory profile in subcutaneous and epicardial adipose tissue in men with and without diabetes. Heart Vessels 2014; 29: 42–48. [DOI] [PubMed] [Google Scholar]
- 66. Mráz M, Cinkajzlová A, Kloučková J, et al. Dendritic cells in subcutaneous and epicardial adipose tissue of subjects with type 2 diabetes, obesity, and coronary artery disease. Mediators Inflamm 2019; 2019: 5481725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Santiago-Fernández C, Pérez-Belmonte LM, Millán-Gómez M, et al. Overexpression of scavenger receptor and infiltration of macrophage in epicardial adipose tissue of patients with ischemic heart disease and diabetes. J Transl Med 2019; 17: 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Sacks HS, Fain JN, Bahouth SW, et al. Adult epicardial fat exhibits beige features. J Clin Endocrinol Metab 2013; 98: E1448–E1455. [DOI] [PubMed] [Google Scholar]
- 69. Sacks HS, Fain JN, Holman B, et al. Uncoupling protein-1 and related messenger ribonucleic acids in human epicardial and other adipose tissues: epicardial fat functioning as brown fat. J Clin Endocrinol Metab 2009; 94: 3611–3615. [DOI] [PubMed] [Google Scholar]
- 70. Dozio E, Vianello E, Briganti S, et al. Increased reactive oxygen species production in epicardial adipose tissues from coronary artery disease patients is associated with brown-to-white adipocyte trans-differentiation. Int J Cardiol 2014; 174: 413–414. [DOI] [PubMed] [Google Scholar]
- 71. Aldiss P, Davies G, Woods R, et al. ‘Browning’ the cardiac and peri-vascular adipose tissues to modulate cardiovascular risk. Int J Cardiol 2017; 228: 265–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Moreno-Santos I, Pérez-Belmonte LM, Macías-González M, et al. Type 2 diabetes is associated with decreased PGC1α expression in epicardial adipose tissue of patients with coronary artery disease. J Transl Med 2016; 14: 243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Iacobellis G. Epicardial and pericardial fat: close, but very different. Obesity (Silver Spring) 2009; 17: 625. [DOI] [PubMed] [Google Scholar]
- 74. Chhabra L, Kowlgi NG. Cardiac adipose tissue: distinction between epicardial and pericardial fat remains important! Int J Cardiol 2015; 201: 274–275. [DOI] [PubMed] [Google Scholar]
- 75. Sacks HS, Fain JN. Human epicardial adipose tissue: a review. Am Heart J 2007; 153: 907–917. [DOI] [PubMed] [Google Scholar]
- 76. Guauque-Olarte S, Gaudreault N, Piché MÈ, et al. The transcriptome of human epicardial, mediastinal and subcutaneous adipose tissues in men with coronary artery disease. PLoS One 2011; 6: e19908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Shah RV, Anderson A, Ding J, et al. Pericardial, but not hepatic, fat by computed tomography is associated with cardiovascular outcomes and structure: the Multi-Ethnic study of Atherosclerosis (MESA). JACC Cardiovasc Imaging 2017; 10: 1016–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Ding J, Hsu FC, Harris TB, et al. The association of pericardial fat with incident coronary heart disease: the Multi-Ethnic study of Atherosclerosis (MESA). Am J Clin Nutr 2009; 90: 499–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Rosito GA, Massaro JM, Hoffmann U, et al. Pericardial fat, visceral abdominal fat, cardiovascular disease risk factors, and vascular calcification in a community-based sample: the Framingham heart study. Circulation 2008; 117: 605–613. [DOI] [PubMed] [Google Scholar]
- 80. Cheng VY, Dey D, Tamarappoo B, et al. Pericardial fat burden on ECG-gated noncontrast CT in asymptomatic patients who subsequently experience adverse cardiovascular events. JACC Cardiovasc Imaging 2010; 3: 352–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Greif M, Leber AW, Saam T, et al. Determination of pericardial adipose tissue increases the prognostic accuracy of coronary artery calcification for future cardiovascular events. Cardiology 2012; 121: 220–227. [DOI] [PubMed] [Google Scholar]
- 82. Yang FS, Yun CH, Wu TH, et al. High pericardial and peri-aortic adipose tissue burden in pre-diabetic and diabetic subjects. BMC Cardiovasc Disord 2013; 13: 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Liberale L, Carbone F, Montecucco F. Pericardial adipose tissue and cardiovascular diseases: new insights from basic research. Eur J Clin Invest 2019; 49: e13052. [DOI] [PubMed] [Google Scholar]
- 84. Sicari R, Sironi AM, Petz R, et al. Pericardial rather than epicardial fat is a cardiometabolic risk marker: an MRI vs echo study. J Am Soc Echocardiogr 2011; 24: 1156–1162. [DOI] [PubMed] [Google Scholar]
- 85. Wang CP, Hsu HL, Hung WC, et al. Increased epicardial adipose tissue (EAT) volume in type 2 diabetes mellitus and association with metabolic syndrome and severity of coronary atherosclerosis. Clin Endocrinol (Oxf) 2009; 70: 876–882. [DOI] [PubMed] [Google Scholar]
- 86. Tonbul HZ, Turkmen K, Kayikcioglu H, et al. Epicardial adipose tissue and coronary artery calcification in diabetic and nondiabetic end-stage renal disease patients. Ren Fail 2011; 33: 770–775. [DOI] [PubMed] [Google Scholar]
- 87. Gaborit B, Kober F, Jacquier A, et al. Assessment of epicardial fat volume and myocardial triglyceride content in severely obese subjects: relationship to metabolic profile, cardiac function and visceral fat. Int J Obes (Lond) 2012; 36: 422–430. [DOI] [PubMed] [Google Scholar]
- 88. Çetin M, Kocaman SA, Durakoĝlugil ME, et al. Effect of epicardial adipose tissue on diastolic functions and left atrial dimension in untreated hypertensive patients with normal systolic function. J Cardiol 2013; 61: 359–364. [DOI] [PubMed] [Google Scholar]
- 89. Rabkin SW. The relationship between epicardial fat and indices of obesity and the metabolic syndrome: a systematic review and meta-analysis. Metab Syndr Relat Disord 2014; 12: 31–42. [DOI] [PubMed] [Google Scholar]
- 90. Iacobellis G, Diaz S, Mendez A, et al. Increased epicardial fat and plasma leptin in type 1 diabetes independently of obesity. Nutr Metab Cardiovasc Dis 2014; 24: 725–729. [DOI] [PubMed] [Google Scholar]
- 91. Chambers MA, Shaibi GQ, Kapadia CR, et al. Epicardial adipose thickness in youth with type 1 diabetes. Pediatr Diabetes 2019; 20: 941–945. [DOI] [PubMed] [Google Scholar]
- 92. Darabian S, Backlund JYC, Cleary PA, et al. Significance of epicardial and intrathoracic adipose tissue volume among type 1 diabetes patients in the DCCT/EDIC: a pilot study. PLoS One 2016; 11: e0159958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Svanteson M, Holte KB, Haig Y, et al. Coronary plaque characteristics and epicardial fat tissue in long term survivors of type 1 diabetes identified by coronary computed tomography angiography. Cardiovasc Diabetol 2019; 18: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Zobel EH, Winther SA, von Scholten BJ, et al. Myocardial flow reserve assessed by cardiac 82Rb positron emission tomography/computed tomography is associated with albumin excretion in patients with type 1 diabetes. Eur Hear J Cardiovasc Imaging 2019; 20: 796–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Kang J, Kim YC, Park JJ, et al. Increased epicardial adipose tissue thickness is a predictor of new-onset diabetes mellitus in patients with coronary artery disease treated with high-intensity statins. Cardiovasc Diabetol 2018; 17: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Ojeda-Peña AC, Amador-Licona N, Rodríguez-Salazar E, et al. Comparison of epicardial fat thickness in diabetic patients compared to non-diabetics with acute myocardial infarction and ST-segment elevation (AMI-STEMI). Gac Med Mex 2016; 152: 345–349. [PubMed] [Google Scholar]
- 97. Vasques ACJ, Souza JRM, Pareja JC, et al. Epicardial and pericardial fat in type 2 diabetes: favourable effects of biliopancreatic diversion. Obes Surg 2015; 25: 477–485. [DOI] [PubMed] [Google Scholar]
- 98. Peraza-Zaldivar JA, Suárez-Cuenca JA, Aceves-Millán R, et al. Pro-atherogenic mediators and subclinical atherogenesis are related to epicardial adipose tissue thickness in patients with cardiovascular risk. J Int Med Res 2017; 45: 1879–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Seker T, Turkoglu C, HarbalIoglu H, et al. The impact of diabetes on the association between epicardial fat thickness and extent and complexity of coronary artery disease in patients with non-ST elevation myocardial infarction. Kardiol Pol 2017; 75: 1177–1184. [DOI] [PubMed] [Google Scholar]
- 100. Chun H, Suh E, Byun AR, et al. Epicardial fat thickness is associated to type 2 diabetes mellitus in Korean men: a cross-sectional study. Cardiovasc Diabetol 2015; 14: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Wang Z, Zhang Y, Liu W, et al. Evaluation of epicardial adipose tissue in patients of type 2 diabetes mellitus by echocardiography and its correlation with intimal medial thickness of carotid artery. Exp Clin Endocrinol Diabetes 2017; 125: 598–602. [DOI] [PubMed] [Google Scholar]
- 102. Yazici D, Özben B, Yavuz D, et al. Epicardial adipose tissue thickness in type 1 diabetic patients. Endocrine 2011; 40: 250–255. [DOI] [PubMed] [Google Scholar]
- 103. Versteylen MO, Takx RAP, Joosen IAPG, et al. Epicardial adipose tissue volume as a predictor for coronary artery disease in diabetic, impaired fasting glucose, and non-diabetic patients presenting with chest pain. Eur Heart J Cardiovasc Imaging 2012; 13: 517–523. [DOI] [PubMed] [Google Scholar]
- 104. Milanese G, Silva M, Bruno L, et al. Quantification of epicardial fat with cardiac CT angiography and association with cardiovascular risk factors in symptomatic patients: from the ALTER-BIO (alternative cardiovascular bio-imaging markers) registry. Diagnostic Interv Radiol 2019; 25: 35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Zobel EH, Christensen RH, Winther SA, et al. Relation of cardiac adipose tissue to coronary calcification and myocardial microvascular function in type 1 and type 2 diabetes. Cardiovasc Diabetol 2020; 19: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Akyürek Ö, Efe D, Kaya Z. Thoracic periaortic adipose tissue in relation to cardiovascular risk in type 2 diabetes mellitus. Wien Klin Wochenschr 2014; 126: 767–773. [DOI] [PubMed] [Google Scholar]
- 107. Rado SD, Lorbeer R, Gatidis S, et al. MRI-based assessment and characterization of epicardial and paracardial fat depots in the context of impaired glucose metabolism and subclinical left-ventricular alterations. Br J Radiol 2019; 92: 20180562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. van Woerden G, Gorter TM, Westenbrink BD, et al. Epicardial fat in heart failure patients with mid-range and preserved ejection fraction. Eur J Heart Fail 2018; 20: 1559–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Gullaksen S, Funck KL, Laugesen E, et al. Volumes of coronary plaque disease in relation to body mass index, waist circumference, truncal fat mass and epicardial adipose tissue in patients with type 2 diabetes mellitus and controls. Diab Vasc Dis Res 2019; 16: 328–336. [DOI] [PubMed] [Google Scholar]
- 110. Al-Talabany S, Mordi I, Houston JG, et al. Epicardial adipose tissue is related to arterial stiffness and inflammation in patients with cardiovascular disease and type 2 diabetes. BMC Cardiovasc Disord 2018; 18: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Mancio J, Oikonomou EK, Antoniades C. Perivascular adipose tissue and coronary atherosclerosis. Heart 2018; 104: 1654–1662. [DOI] [PubMed] [Google Scholar]
- 112. Mahabadi AA, Massaro JM, Rosito GA, et al. Association of pericardial fat, intrathoracic fat, and visceral abdominal fat with cardiovascular disease burden: the Framingham heart study. Eur Heart J 2009; 30: 850–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Mahabadi AA, Lehmann N, Möhlenkamp S, et al. Noncoronary measures enhance the predictive value of cardiac CT above traditional risk factors and CAC score in the general population. JACC Cardiovasc Imaging 2016; 9: 1177–1185. [DOI] [PubMed] [Google Scholar]
- 114. Mahabadi AA, Lehmann N, Kälsch H, et al. Association of epicardial adipose tissue and left atrial size on non-contrast CT with atrial fibrillation: the Heinz Nixdorf recall Study. Eur Heart J Cardiovasc Imaging 2014; 15: 863–869. [DOI] [PubMed] [Google Scholar]
- 115. Mahabadi AA, Lehmann N, Kälsch H, et al. Association of epicardial adipose tissue with progression of coronary artery calcification is more pronounced in the early phase of atherosclerosis: results from the Heinz Nixdorf recall study. JACC Cardiovasc Imaging 2014; 7: 909–916. [DOI] [PubMed] [Google Scholar]
- 116. Mahabadi AA, Berg MH, Lehmann N, et al. Association of epicardial fat with cardiovascular risk factors and incident myocardial infarction in the general population: the Heinz Nixdorf recall study. J Am Coll Cardiol 2013; 61: 1388–1395. [DOI] [PubMed] [Google Scholar]
- 117. Nerlekar N, Brown AJ, Muthalaly RG, et al. Association of epicardial adipose tissue and high-risk plaque characteristics: a systematic review and meta-analysis. J Am Heart Assoc 2017; 6: e006379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Mancio J, Azevedo D, Saraiva F, et al. Epicardial adipose tissue volume assessed by computed tomography and coronary artery disease: a systematic review and meta-analysis. Eur Heart J Cardiovasc Imaging. 2018; 19: 490–497. [DOI] [PubMed] [Google Scholar]
- 119. Reinhardt M, Cushman TR, Thearle MS, et al. Epicardial adipose tissue is a predictor of decreased kidney function and coronary artery calcification in youth- and early adult onset type 2 diabetes mellitus. J Endocrinol Invest 2019; 42: 979–986. [DOI] [PubMed] [Google Scholar]
- 120. Kim HM, Kim KJ, Lee HJ, et al. Epicardial adipose tissue thickness is an indicator for coronary artery stenosis in asymptomatic type 2 diabetic patients: its assessment by cardiac magnetic resonance. Cardiovasc Diabetol 2012; 11: 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Chen WJY, Danad I, Raijmakers PG, et al. Effect of type 2 diabetes mellitus on epicardial adipose tissue volume and coronary vasomotor function. Am J Cardiol 2014; 113: 90–97. [DOI] [PubMed] [Google Scholar]
- 122. Yerramasu A, Dey D, Venuraju S, et al. Increased volume of epicardial fat is an independent risk factor for accelerated progression of sub-clinical coronary atherosclerosis. Atherosclerosis 2012; 220: 223–230. [DOI] [PubMed] [Google Scholar]
- 123. Kazlauskaite R, Doukky R, Evans A, et al. Predictors of diastolic dysfunction among minority patients with newly diagnosed type 2 diabetes. Diabetes Res Clin Pract 2010; 88: 189–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Levelt E, Pavlides M, Banerjee R, et al. Ectopic and visceral fat deposition in lean and obese patients with type 2 diabetes. J Am Coll Cardiol 2016; 68: 53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Uygur B, Celik O, Ozturk D, et al. The relationship between location-specific epicardial adipose tissue volume and coronary atherosclerotic plaque burden in type 2 diabetic patients. Kardiol Pol 2017; 75: 204–212. [DOI] [PubMed] [Google Scholar]
- 126. Maimaituxun G, Shimabukuro M, Fukuda D, et al. Local thickness of epicardial adipose tissue surrounding the left anterior descending artery is a simple predictor of coronary artery disease - new prediction model in combination with Framingham risk score. Circ J 2018; 82: 1369–1378. [DOI] [PubMed] [Google Scholar]
- 127. Oikonomou EK, Marwan M, Desai MY, et al. Non-invasive detection of coronary inflammation using computed tomography and prediction of residual cardiovascular risk (the CRISP CT study): a post-hoc analysis of prospective outcome data. Lancet 2018; 392: 929–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Ansaldo AM, Montecucco F, Sahebkar A, et al. Epicardial adipose tissue and cardiovascular diseases. Int J Cardiol 2019; 278: 254–260. [DOI] [PubMed] [Google Scholar]
- 129. Topuz M, Dogan A. The effect of epicardial adipose tissue thickness on left ventricular diastolic functions in patients with normal coronary arteries. Kardiol Pol 2017; 75: 196–203. [DOI] [PubMed] [Google Scholar]
- 130. Nakanishi K, Fukuda S, Tanaka A, et al. Persistent epicardial adipose tissue accumulation is associated with coronary plaque vulnerability and future acute coronary syndrome in non-obese subjects with coronary artery disease. Atherosclerosis 2014; 237: 353–360. [DOI] [PubMed] [Google Scholar]
- 131. Doesch C, Haghi D, Flüchter S, et al. Epicardial adipose tissue in patients with heart failure. J Cardiovasc Magn Reson 2010; 12: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Park HE, Choi SY, Kim M. Association of epicardial fat with left ventricular diastolic function in subjects with metabolic syndrome: assessment using 2-dimensional echocardiography. BMC Cardiovasc Disord 2014; 14: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Fontes-Carvalho R, Fontes-Oliveira M, Sampaio F, et al. Influence of epicardial and visceral fat on left ventricular diastolic and systolic functions in patients after myocardial infarction. Am J Cardiol 2014; 114: 1663–1669. [DOI] [PubMed] [Google Scholar]
- 134. Watanabe K, Kishino T, Sano J, et al. Relationship between epicardial adipose tissue thickness and early impairment of left ventricular systolic function in patients with preserved ejection fraction. Heart Vessels 2016; 31: 1010–1015. [DOI] [PubMed] [Google Scholar]
- 135. Cheng VY. Plugging epicardial fat into a prediction algorithm. Circ Cardiovasc Imaging 2019; 12: e008629. [DOI] [PubMed] [Google Scholar]
- 136. Davidovich D, Gastaldelli A, Sicari R. Imaging cardiac fat. Eur Heart J Cardiovasc Imaging 2013; 14: 625–630. [DOI] [PubMed] [Google Scholar]
- 137. González N, Moreno-Villegas Z, González-Bris A, et al. Regulation of visceral and epicardial adipose tissue for preventing cardiovascular injuries associated to obesity and diabetes. Cardiovasc Diabetol 2017; 16: 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Liu CY, Redheuil A, Ouwerkerk R, et al. Myocardial fat quantification in humans: evaluation by two-point water-fat imaging and localized proton spectroscopy. Magn Reson Med 2010; 63: 892–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Iacobellis G. Epicardial fat: a new cardiovascular therapeutic target. Curr Opin Pharmacol 2016; 27: 13–18. [DOI] [PubMed] [Google Scholar]
- 140. Sato T, Aizawa Y, Yuasa S, et al. The effect of dapagliflozin treatment on epicardial adipose tissue volume. Cardiovasc Diabetol 2018; 17: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Yagi S, Hirata Y, Ise T, et al. Canagliflozin reduces epicardial fat in patients with type 2 diabetes mellitus. Diabetol Metab Syndr 2017; 9: 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. van Eyk HJ, Paiman EHM, Bizino MB, et al. A double-blind, placebo-controlled, randomised trial to assess the effect of liraglutide on ectopic fat accumulation in South Asian type 2 diabetes patients. Cardiovasc Diabetol 2019; 18: 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Iacobellis G, Mohseni M, Bianco SD, et al. Liraglutide causes large and rapid epicardial fat reduction. Obesity (Silver Spring) 2017; 25: 311–316. [DOI] [PubMed] [Google Scholar]
- 144. Rabkin SW, Campbell H. Comparison of reducing epicardial fat by exercise, diet or bariatric surgery weight loss strategies: a systematic review and meta-analysis. Obes Rev 2015; 16: 406–415. [DOI] [PubMed] [Google Scholar]
- 145. Jonker JT, de Mol P, de Vries ST, et al. Exercise and type 2 diabetes mellitus: changes in tissue-specific fat distribution and cardiac function. Radiology 2013; 269: 434–442. [DOI] [PubMed] [Google Scholar]
- 146. Gonzalez GF, Rodriguez MAR, Pareja MAR, et al. A home-based treadmill training reduced epicardial and abdominal fat in postmenopausal women with metabolic syndrome. Nutr Hosp 2014; 30: 609–613. [DOI] [PubMed] [Google Scholar]
- 147. Christensen RH, Wedell-Neergaard AS, Lehrskov LL, et al. Effect of aerobic and resistance exercise on cardiac adipose tissues: secondary analyses from a randomized controlled trial. JAMA Cardiol 2019; 4: 778–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Christensen RH, Lehrskov LL, Wedell-Neergaard AS, et al. Aerobic exercise induces cardiac fat loss and alters cardiac muscle mass through an interleukin-6 receptor-dependent mechanism: cardiac analysis of a double-blind randomized controlled clinical trial in abdominally obese humans. Circulation. 2019; 140: 1684–1686. [DOI] [PubMed] [Google Scholar]
- 149. Packer M. Disease-treatment interactions in the management of patients with obesity and diabetes who have atrial fibrillation: the potential mediating influence of epicardial adipose tissue. Cardiovasc Diabetol 2019; 18: 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Iacobellis G, Mahabadi AA. Is epicardial fat attenuation a novel marker of coronary inflammation? Atherosclerosis 2019; 284: 212–213. [DOI] [PubMed] [Google Scholar]
- 151. Patel VB, Shah S, Verma S, et al. Epicardial adipose tissue as a metabolic transducer: role in heart failure and coronary artery disease. Heart Fail Rev 2017; 22: 889–902. [DOI] [PubMed] [Google Scholar]
- 152. Nedergaard J, Bengtsson T, Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab 2007; 293: E444–E452. [DOI] [PubMed] [Google Scholar]
- 153. Zhou J, Chen Y, Zhang Y, et al. Epicardial fat volume improves the prediction of obstructive coronary artery disease above traditional risk factors and coronary calcium score. Circ Cardiovasc Imaging 2019; 12: e008002. [DOI] [PubMed] [Google Scholar]