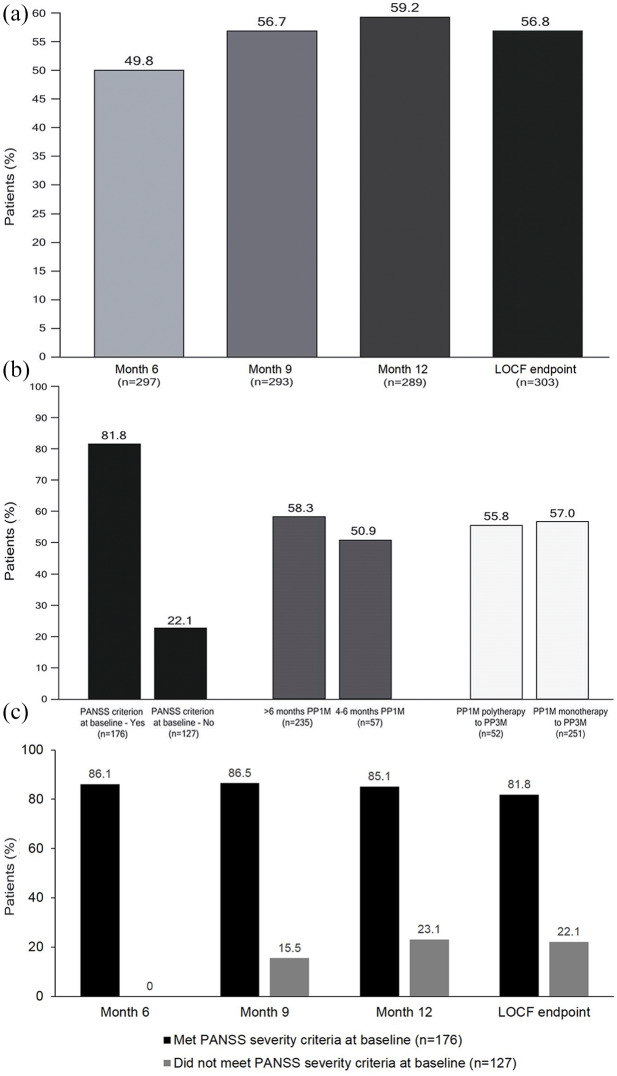
Figure 3.
Achievement of SR during the treatment period in the whole population over time (A) and in subgroups at LOCF endpoint: PANSS remission symptom severity criterion at baseline – yes versus no; >6 months prior PP1M versus 4–6 months prior PP1M; and switched from PP1M polytherapy versus PP1M monotherapy (B), and in patients who met and did not meet the PANSS severity criterion for remission at baseline (C) (mITT population).
LOCF, last observation carried forward; mITT, modified intent-to-treat; PANSS, positive and negative syndrome scale; PP1M, paliperidone palmitate 1-month formulation; SR, symptomatic remission.