Abstract
Antimicrobial de-escalation (ADE) is a component of antimicrobial stewardship (AMS) aimed to reduce exposure to broad-spectrum antimicrobials. In the intensive care unit, ADE is a strong recommendation that is moderately applied in clinical practice. Following a systematic review of the literature, we assessed the studies identified on the topic which included one randomized controlled trial and 20 observational studies. The literature shows a low level of evidence, although observational studies suggested that this procedure is safe. The effects of ADE on the level of resistance of ecological systems and especially on the microbiota are unclear. The reviewers recommend de-escalating antimicrobial treatment in patients requiring long-term antibiotic therapy and considering de-escalation in short-term treatments.
Electronic supplementary material
The online version of this article (10.1007/s12325-020-01390-2) contains supplementary material, which is available to authorized users.
Keywords: Antibiotic, Antimicrobial, Documentation, Infectious disease, Multidrug resistance, Stewardship
Key Summary Points
| Antibiotic de-escalation (ADE) was first implemented to reduce exposition to broad-spectrum antibiotics in the ICU. It is now part of the antimicrobial stewardship and is recommended in international guidelines. |
| This systematic review aimed to evaluate the level of evidence regarding the safety of ADE (mortality, superinfections and duration of antimicrobial therapy) and the emergence of antibiotic resistance. |
| Data related to safety showed with a low evidence level to support the safety of ADE. No conclusion can be drawn on the level of resistance after de-escalation. |
| The reviewers recommend de-escalating antimicrobial treatment in patients requiring long-term antibiotic therapy and discussing the need for de-escalation for patients requiring short-term treatments. |
Introduction
Antimicrobial de-escalation (ADE) is a component of antimicrobial stewardship (AMS) aimed to reduce exposure to broad-spectrum antimicrobials. Broad-spectrum antimicrobials are widely used in intensive care units (ICUs), often empirically in the treatment of patients with life-threatening infections [1].
The Surviving Sepsis Campaign guidelines [2] recommend early administration of broad-spectrum antimicrobials to patients with sepsis. This may be achieved with administration of a pivotal antibiotic, most often a beta-lactam, accompanied by an additional antimicrobial intended to extend the spectrum, create a synergy between some antibiotics and reduce the emergence of antimicrobial resistance. This recommendation was strong and clear despite being based solely on observational studies reporting associations between delays in antimicrobial administration and mortality rates. However, it was not universally supported by the entire scientific community, notably the Infectious Diseases Society of America [3]. The Surviving Sepsis Campaign guidelines also recommended daily assessment for possible ADE in these patients. Lacking evidence, this recommendation was classified as a best practice statement. The current paper reviews the definition, the principles and the ecological consequences of ADE.
Method
For the purpose of this review we conducted a systematic search of the Pubmed database from January 1, 2005 until January 15, 2020 for studies on ICU patients (P) describing de-escalation of antibiotic therapy (I) versus continuation without de-escalation (C) in relation to either patient outcomes or antimicrobial resistance profiles (O). The study was registered in PROSPERO (CRD42020169433). Details regarding the search methodology, study inclusion criteria and method used to assess the quality of the included studies are provided in the supplementary material.
We identified one randomized controlled trial (RCT) [4] and 20 observational studies describing ADE in ICU patients [5–24]. We excluded studies evaluating antifungal de-escalation alone as well as case reports, case series and reviews. We also excluded papers not in the English language and/or those conducted on animal models or the paediatric population. The 20 observational papers were assessed for quality using the Newcastle Outcome Score (Table 3). This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Table 3.
Summary of included studies
| Studies | Study quality grade (mean) | No. patients | Mortality (%) | Length of stay (days) | Superinfections % | Severity score | AMR emergence % | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ADE | No ADE | p | ADE | No ADE | p | ADE | No ADE | p | ADE | No ADE | p | ADE | No ADE | p | |||
| Alvarez-Lerma 2006 [5] | 5 | 14.5% | 20% | – | 23.7 | 25.4 | 0.46 | – | – | – | 16.0a | 17.4a | – | – | – | – | |
| Giantsou 2007 [6] | 4 | 113 | 12.0% | 43.5% | < 0.05 | 17.2 (1.2)d | 22.7 (6.3)d | < 0.05 | – | – | – | 7.1 (1.09)c | 7.1 (1.1)c | 0.9 | – | – | – |
| Eachempati 2009 [17] | 4 | 138 | 33.8% | 42.1% | 0.324 | – | – | – | 27.30% | 35.10% | 0.34 | 79.8 (3.2)a | 85.5 (3.1)a | 0.223 | – | – | – |
| De Waele 2010 [18] | 5 | 113 | 7%d | 21%d | 0.12 | – | – | – | – | – | – | 19(6.8)a | 18 (5.0)a | 0.87 | – | – | – |
| Morel 2010 [19] | 6 | 116 | 18.3% | 24.6% | NS | 28 (33) | 24 (23) | 0.38 | 5% | 19% | 0.01 | 41(15)b | 40 (16)b | 0.68 | 10% | 19.1% | 0.10 |
| Joung 2011 [20] | 6 | 137 | 2.3% | 14.0% | 0.03 | – | – | – | – | – | – | 15.6 (5.5)a | 15.3 (5.3)a | 0.90 | – | – | – |
| Heenen 2012 [21] | 5 | 169 | 16% | 26% | 0.20e | – | – | – | – | – | – | – | – | – | – | – | – |
| Gonzalez 2013 [22] | 7 | 229 | 17.1%d | 18.7%d | 0.5 | 12.9 (15.6)d | 10 (12.9)d | 0.12 | 2.50% | 5.30% | 0.15 | 7.8 (4.6)c | 7.2 (4.5)c | 0.32 | 15.30% | 10.70% | 0.1 |
| Knaak 2013 [23] | 5 | 113 | 15%f | 39%f | < 0.01 | 15.4 (15.3)f | 18.0 (12.9)f | 0.35 | – | – | – | 5.7(3.3)c | 5.3 (3.7)c | 0.44 | – | – | – |
| Mokart 2013 [24] | 6 | 101 | 18.18% | 26.32% | 0.57 | – | – | – | – | – | – | 8.5 [6;10.25]c | 7 [5;10]c | 0.37 | – | – | – |
| Garnacho-Montero 2013 [7] | 7 | 628 | 28.3% | 34.1% | 0.001 | 26 (15–40)f | 24 (15–38)f | 0.015 | – | – | – | 7(4–10)c | 7 (4–10)c | 0.60 | – | – | – |
| Paskovaty 2015 [8] | 5 | 101 | 24 (39%) | 15 (34%) | 0.68 | 17.1 (22.9)f | 23.4 (17.6)f | 0.005 | – | – | – | 7.2 (± 3.3)c | 8 (± 3.4)c | 0.18 | – | – | – |
| Moraes 2016 [9] | 4 | 224 | 56.8% | 56.10% | 0.99 | 21 (10–37) | 19.5 (10–40) | > 0.05 | – | – | – | 7.9 ( 3.6)c | 7.3 (3.8)c | > 0.05 | – | – | – |
| Weiss 2016 [10] | 5 | 182 | 31% | 26% | 0.53 | 16 (10.21) | 18 (12.21) | 0.82 | – | – | – | 6 [3;9]c | 5 [3;8]c | 0.46 | 14.30% | 21.30% | 0.32 |
| De Bus 2016 [11] | 5 | 418 | 22.4%d | 21.3%d | 0.84 | 11 [6;19]d | 8 [5;15]d | 0.001 | 38.80% | 33% | 0.34 | 23 [18;30]a | 22 [17;28]a | 0.31 | 28.20% | 27.60% | 0.91 |
| 32.9%f | 33%f | 0.99 | |||||||||||||||
| Turza 2016 [12] | 4 | 2658 | 6% | 9% | 0.002 | – | – | – | – | – | – | 15 (8)a | 14 (8)a | 0.02 | – | – | – |
| Trupka 2017 [13] | 4 | 283 | 16.4%f | 43.3%f | < 0.001 | 11.0 [6.0;22.0]f | 12.0 [6.0;20]f | 0.918 | 8.3%g | 8.6%g | 0.92 | 22.7 (7.3)a | 21.7 (7.8)a | 0.262 | 6.3%h | 4.3%h | 0.468 |
| Khan 2017 [14] | 5 | 108 | 21.9%d | 23.7%d | NS | 10.1 (4.6)d | 10.3 (9.1)d | NS | – | – | – | 10 (3.3)c | 9.8 (2.8)c | NS | – | – | – |
| Li 2018 [15] | 6 | 156 | 28.6% | 23.80% | 0.620 | 19 [15;23] | 19 [15;26] | 0.764 | – | – | – | 16 [14;20] | 15.5 [14;19] | 0.346 | 31.0% | 40.5% | 0.36 |
| Cowley 2019 [16] | 4 | 279 | 22.8% | 28.3% | 0.39 | 10 (5.24)d | 13 (8.23)d | Sig | – | – | – | 13 [10;18]a | 13 [9;17]a | NS | – | – | – |
| 15 [8;30]f | 20 [11;34]f | Sig | – | – | – | ||||||||||||
ADE antimicrobial de-escalation, AMR antimicrobial resistance, NS not significant, Sig significant
aAPACHE (Acute Physiology and Chronic Health Evaluation)
bSAPS (Simplified Acute Physiology Score)
cSOFA (Sequential Organ Failure Assessment)
dLOS (length of stay) or mortality in ICU
eFisher exact test calculated a posteriori
fLOS or mortality in hospital
gOnly secondary pneumonia considered as superinfection event
hAMR emergence considered here as secondary pneumonia due to resistant pathogens
Principles
The goals of ADE and the actions that may be considered ADE are listed in Table 1. The main criterion used to determine whether successive use of different antibiotics can be considered de-escalation is the ranking of the spectrum of antimicrobial coverage of these antibiotics. Two reports have attempted to rank antibiotics directed against Gram-negative bacteria, resulting in divergent findings (Table 2). Madaras-Kelly et al. scored antibiotic regimens on the basis of the susceptibilities of different microorganisms of clinical interest to each regimen [25]. Using this computed antibiotic regimen score they then ranked a total of 27 antibiotics ranging from a minimum score of 4 (metronidazole) to a maximum score of 49.75 (tigecycline). The authors then asked a group of experts to determine whether specific changes in antibiotic regimen are ADEs or not. The responses of the experts did not correlate well with ADEs as defined by the calculated scores, underscoring the complexity of bench to bedside translation of ADE. Weiss et al. used a Delphi process to rank six β-lactam antibiotics according to their spectrum and their ecological impact on the microbiota [26]. The Delphi process consisted of obtaining consensus based on the results of multiple rounds of questionnaires sent to a panel of experts. Ranking of ureido/carboxy-penicillins, and third- and fourth-generation cephalosporins needed additional voting rounds as there was less agreement between the voters on these drugs than there was on other molecules. This lack of consensus is probably mostly attributable to a lack of data regarding the effect of these molecules on the microbiota [26], but it can also be related to the fact that the effects of antibiotics depend on the ecosystem (ICU, hospital, country) where they are used. Furthermore, as shown in Table 2, the two scores are also significantly different. At this time, most experts recommend that each institution creates its own ranking based on local epidemiology [1] and antimicrobial availability.
Table 1.
Goals and actions considered ADE (antimicrobial de-escalation)
| The goals of therapy are theoretically [3] |
| Broadening the spectrum of antimicrobial therapy by administering different agents acting on different families of pathogens. This increases the likelihood that any responsible pathogen will be susceptible to at least one of the administered agents |
| Improving the lethality of the treatment on the basis of a possible synergistic effect |
| Preventing or delaying the emergence of resistance |
| A large number of actions can be considered ADE, making it challenging to reach a consensus |
| Narrowing the spectrum of the pivotal antimicrobial |
| Early discontinuation of one or several antimicrobials of a combination therapy |
| Early discontinuation of antimicrobial treatment. This has been excluded from the definition of ADE in the last consensus statement [1] |
Table 2.
Differences in antibiotic ranking according to Weiss et al. and Madaras-Kelly et al.
| Weiss et al. | Agent | Madaras-Kelly et al. | ||
|---|---|---|---|---|
| Rank | Similar response (%)a | Spectrum score | Rank | |
| 1 | 100 | Amoxicillin | 13.5 | 1 |
| 2 | 88 | Amoxicillin/clavulanate | 29.5 | 3 |
| 3 | 81 | 3rd-generation cephalosporin | 25.5 | 2 |
| 4 | 71 | Piperacillin/tazobactam | 42.25 | 7 |
| 4th-generation cephalosporin | 33.25 | 5 | ||
| 5 | 81 | Ertapenem | 30.25 | 4 |
| 6 | 85 | Imipenem | 41.5 | 6 |
aIndicates the proportion of experts that agreed with the molecules included in each rank of the classification
Definition
In order to best define ADE, international experts (n = 16) were requested to define what constitutes ADE in their opinion. The experts were also asked their opinion as to how ADE should be implemented in clinical practice [1]. Expert selection was based on prior publications in the field of antimicrobial stewardship, experience with systematic reviews and group writing. Thirteen issues related to the definition of ADE were put forward by the participants. Lacking data, a Delphi method was used to reach expert consensus regarding the final version of the definition. Votes were conducted anonymously, in a blinded fashion, and occurred after collective discussion of each proposal. The number of allowable voting rounds was pre-set to a maximum of three and consensus was predefined as 70% agreement. The final definition of ADE decided upon comprised the following:
Replacing administration of broad-spectrum antimicrobials with agents having a narrower spectrum of antimicrobial coverage or local ecological impact.
-
Stopping administration of components of an antimicrobial combination. Two different situations were included in this definition.
- Stopping administration of an antimicrobial agent administered as a component of combination therapy in order to provide double coverage for certain pathogens.
- Stopping administration of an antimicrobial agent given empirically, following verification that the covered pathogens have not been isolated in clinical cultures.
Early discontinuation of all antimicrobial therapy once infection has been ruled out is not considered de-escalation [1].
Patient Perspective: Safety of Antimicrobial De-escalation
Data on patient outcomes and the safety of ADE are reported in Table 3.
Association of ADE with Mortality
The non-blinded RCT published by Leone et al. was a multicentre non-inferiority trial assessing ADE versus continuation of empirical treatment in 120 patients. The study showed that for the primary endpoint—ICU length of stay—ADE did not reach the margin of non-inferiority compared with continuation (15.2 ± 15.0 days for ADE versus 11.8 ± 12.6 days for continuation, p = 0.71, non-inferiority margin 2 days). No difference was also observed in 28-day mortality (31% with ADE group versus 23% with treatment continuation, p = 0.55) [4].
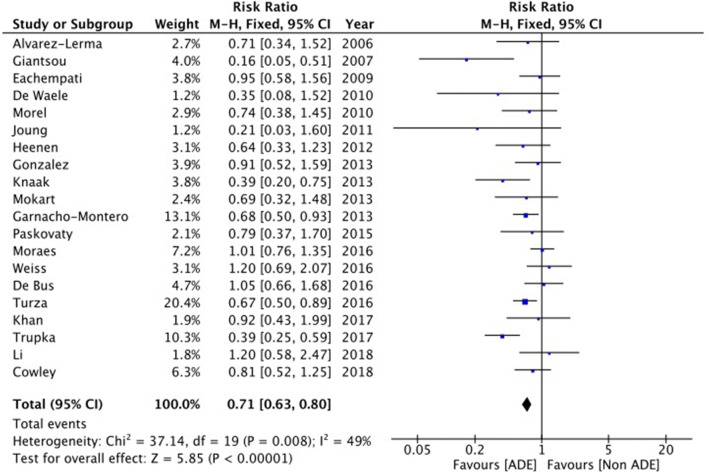
Among the 20 observational studies, differences in mortality between ADE and non-ADE were found to be not statistically significant in 14 studies and significantly decreased in the de-escalation arm in six studies [6, 7, 12, 13, 20, 23]. None showed an increased mortality associated with ADE. Data synthesis of the observational studies suggests that ADE is associated with lower mortality with a relative risk at 0.71 and a 95% confidence interval from 0.63 to 0.80, but substantial heterogeneity was identified between studies with regards to this outcome (I2 = 49%) (Fig. 1).
Fig. 1.
Difference in patient mortality rates between antimicrobial de-escalation (ADE) and non-ADE
Definition of ADE was highly variable in observational studies; therefore, a sensitivity analysis was further performed to assess only studies not including early antibiotic cessation in the definition of ADE. The sensitivity analysis found a relative risk at 0.67 with a 95% confidence interval from 0.59 to 0.76 (details and forest plot in supplementary material). However, the data were not adjusted in any of the studies.
Association of ADE with Superinfections and Duration of Antimicrobial Therapy
The RCT showed that ADE was associated with a higher number of superinfections and a subsequent increase in duration of antimicrobial therapy compared to continuation (27% in ADE versus 11% in non-ADE, p = 0.03). However, the trial was not powered for this outcome.
Six observational studies, which did not include early cessation of antimicrobials in the definition of ADE, reported on these outcomes (n trials = 6, n patients = 1444). Three showed similar durations of antibiotic treatment [5, 10, 13], two showed an increase in duration with ADE [11, 24] and one showed a decrease in duration with ADE [15].
Two reasons were put forward for the increased duration of treatment observed in two of the studies. These included superinfections and mistaken perceptions that prolonged treatment are reasonable with narrow-spectrum but not broad-spectrum antibiotics. This finding prompted a recommendation by the authors of this paper that clinicians should consider limiting the duration of antimicrobial therapy regardless of the spectrum of antibiotic coverage. Shortening the duration of antibiotic therapy is one of the strategies proven to decrease antibiotic resistance [27].
A topic not addressed in any of the papers was the risk of inadequate dosing during ADE. Carlier et al. showed that the probability of reaching pharmacokinetic/pharmacodynamic (PK/PD) targets during de-escalating from a broad-spectrum antimicrobial to a narrow-spectrum antimicrobial is low when using usual antimicrobial doses [28]. Recommendations for narrow-spectrum antibiotics dosage are identical for ICU and non-ICU patients. Yet, ICU patients commonly have altered distribution volumes and renal function. Therefore, PK/PD adjustments are probably needed during ADE, as recommended for all antimicrobial treatment [29].
In brief, the only existing RCT included a small number of patients and it was not powered to meet some of the important endpoints. The observational studies have a significant risk of inclusion bias; patients treated with ADE were mostly less severe than those continuing antibiotic treatment [21] (Table 3). Even with the use of statistical adjustment such as logistic or linear regression analysis [5, 8, 10, 22], and propensity score matching [7, 15], the risk of bias remains [30]. In observational studies, several risk factors for not implementing ADE were found including high patient’s severity [7, 8, 12, 15, 20, 23], identification of a multidrug-resistant (MDR) pathogen in the cultures [5, 11, 13, 18, 19, 22], presence of multiple pathogens in the cultures [7, 8, 23], a non-microbiologically documented infection [11, 14] or infections with a high risk of undiagnosed pathogens such as intra-abdominal infections [7, 12, 18]. Finally, ADE may reflect better management by the physician in charge.
To date, the lack of high-quality data hampers provision of strong recommendations. The authors therefore chose to adhere to the recently published position statement on ADE which suggested that ADE may be safe in most patients if the practice is included in a global antimicrobial stewardship program [1]. At this time three RCTs are ongoing on this topic. These studies are recruiting patients with bacteraemia, malignancy and sepsis or septic shock and infections in the ICU (overall n = 1460) [31–33]. It is hoped that their additional data will enable one to draw conclusions on this issue.
Epidemiological Perspective: De-escalation and Emergence of Antibiotic Resistance
ADE was introduced to reduce the emergence of antimicrobial resistance (AMR) [2]. The evidence regarding the relation between de-escalation and emergence of AMR can be divided into direct and indirect evidence. The former relates to studies showing an association between ADE and resistance in human subjects. The latter relates to recent evidence on the effect of antimicrobials on the microbiome [34].
Direct Evidence
At this time the evidence does not show the presence of an association between ADE and decreased emergence of MDR. De Bus et al. [11] retrospectively assessed observational data, seeking emergence of antimicrobial resistance after ADE in infected ICU patients treated with anti-pseudomonal β-lactams. They found no significant differences in the emergence of MDR bacteria at day 14 (23.5% in the de-escalation group versus 18.6% in the continuation group, p = 0.2). Leone et al. assessed emergence of MDR bacteria on day 8 as a secondary endpoint in their RCT and showed no difference between fixed treatment and de-escalation [4].
A cohort study of adults with severe sepsis or septic shock (n = 7118) showed that each additional day of exposure to cefepime, meropenem and piperacillin-tazobactam was associated with increased development of resistance [35]. However, three other retrospective observational studies, which assessed antimicrobial resistance as secondary endpoints, identified no statistically significant association between ADE and resistance emergence [10, 15, 22]. The first assessed ADE in septic ICU patients (n = 229). ADE was performed in 51% of patients. The incidence of emergence of MDR bacteria at the time of last ICU screening was 15.3% with ADE versus 10.7% with continued treatment (p = 0.1). In this study, ADE led to more MDR infections, but the difference remains not statistically significant [22]. The second studied ICU patients with ventilator-associated pneumonia (n = 182). ADE was performed in 38% of cases. The incidence of emergence of MDR bacteria by day 21 was 14.3% with ADE versus 21.3% with continued treatment (p = 0.3) [10]. The third study compared patients matched with propensity scoring (n = 84) and showed that the incidence of emergence of antimicrobial resistance during ICU admission was 31% with ADE versus 40.5% with continued treatment (p = 0.3) [15].
Indirect Evidence
The intestinal microbiota is an independent organ wherein MDR bacteria may emerge [34]. The microbiota is a complex ecosystem, containing between 1012 and 1014 bacteria per gram of stool. The extent and complexity of the microbiota are such that the metaphor of “black matter” has been used to describe how little we know about it [36]. Several techniques are currently being developed to assess the microbiota. Among these, metagenomics, also called Next Generation Sequencing (NGS), is increasingly being used in clinical settings [37]. NGS has several advantages over traditional methods. One is that it obviates the need for bacterial culture, which allows detection of fastidious bacteria. Another advantage of NGS is that it assesses the full antimicrobial resistome (i.e. the presence and interaction of genes encoding antimicrobial resistance in a given microbiome) [38]. The combination of these advantages is of particular interest; the microbiota of the human gut is a hub for horizontal exchange of genetic material between bacteria [39]. Therefore transfer of genetic material conferring antimicrobial resistance to non-pathogenic bacteria may be as harmful as its presence in pathogenic bacteria [40]. NGS detects the presence of antimicrobial resistance genes (thereby mapping their potential deleterious effects), regardless of the bacteria hosting those genes.
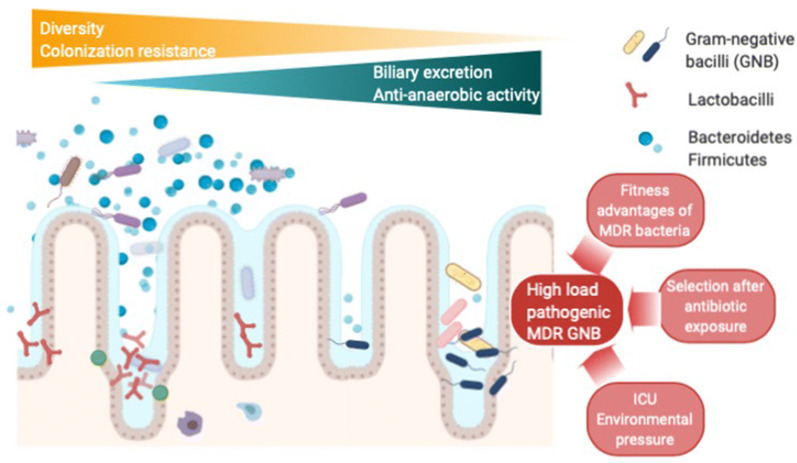
As mentioned above, ADE was first implemented on the basis of the hypothesis that reducing the duration of exposure to broad-spectrum antimicrobials would lead to less emergence of antimicrobial resistance. Woerther et al. reported that the administration of imipenem may have little effect on the diversity of the microbiota and its colonization resistance, despite the fact that imipenem is a broad-spectrum antibiotic [41]. Other antimicrobials, such as piperacillin/tazobactam or ceftriaxone, have been implicated in greater detrimental effects on the gut microbiota. The causes put forward for this difference include antimicrobial features such as the degree of biliary excretion, the spectrum of anti-anaerobic activity and/or the extent of accumulation in the gastrointestinal tract. All of these could potentially lead to disruption of the normal bacterial balance with resultant disturbances in colonization resistance, diversity and balance between phyla [34, 41]. Disruption of colonization resistance is of concern because in the vaccum created, colonization is more likely to occur with MDR bacteria (Fig. 2). Nevertheless, ceftriaxone and piperacillin/tazobactam are often considered for de-escalation from carbapenems [34].
Fig. 2.
Antimicrobial induced alterations of the gut microbiota
Furthermore, even if ADE reduces the duration of exposure to broad-spectrum antibiotics, detrimental effects on microbiota can still occur in a short period of time. Armand-Lefèvre et al. showed that ICU patients exposed to 1–3 days of imipenem had an increased risk of emergence of imipenem-resistant Gram-negative bacilli (odds ratio 5.9, 95% CI 1.5–25.7) [42]. Therefore, especially for short duration treatments, a brief exposure to broad-spectrum antimicrobials followed by a reduction in the antimicrobial spectrum may result in a cumulative effect of different lines of antimicrobials rather than a reduction in the impact on resistances.
Studies have assessed the resistome in ICU patients exposed to antibiotics [43, 44] and in animals exposed to antibiotics [45]. Buelow et al. [43] compared the resistomes of ICU patients and healthy volunteers exposed to antibiotics in the setting of selective digestive tract decontamination (SDD). Ten patients received antimicrobials for SDD: cefotaxime, polymyxin E (colistin), tobramycin and amphotericin B. The resistome of ICU-SDD treated patients differed from that of healthy volunteers and the microbiota of ICU patients was less diverse than that of healthy volunteers. Cessation of SDD and ICU discharge were associated with recolonization by MDR bacteria, confirming the hypothesis that a less diverse microbiota is less resistant to colonization in ICU patients. The integrity of the microbiota is crucial in the battle against bacterial colonization; antibiotic-associated destruction of one or more phyla could provide the opening for persistent installation of MDR pathogens.
Willmann et al. [44] also assessed the resistome of ICU patients in relation to the type of administered antibiotics. The resistomes of hematological patients receiving prophylactic treatment with either cotrimoxazole or ciprofloxacin were compared. The introduction of antibiotics was associated with a substantial increase in antimicrobial resistance gene content. Those treated with cotrimoxazole also showed an increase of antimicrobial resistance genes carried by plasmids (plasmidome). These findings highlight the need for prudent use of antimicrobials and better antibiotic selection according to their impacts on the emergence of bacterial resistance. These studies provide indirect evidence that shuffling antibiotics may have a deleterious cumulative effect on the microbiome as each antibiotic probably has an individual effect on the resistome.
In brief, indirect evidence suggests that reducing exposure to broad-spectrum antimicrobials should be promoted. However, the scarcity of microbiological data does not make it possible to answer the question whether ADE has an effect on the development of antimicrobial resistance. Recent guidelines highlight this lack of evidence [1].
Our Recommendations for Clinical Practice: Expert Opinion
On the basis of the above evidence, we can draw conclusions for the clinical vignette. For infections for which treatment is expected to be short (5–7 days), no benefit has been shown for ADE. Examples include pneumonia, uncomplicated peritonitis and urinary tract infections. If antibiotic susceptibility is known by day 3, whether the antibiotics should be changed is matter for discussion. It is acceptable in such cases to continue the same antibiotic rather than exposing the patient to an additional antibiotic with a whole different spectrum of coverage and a different effect on the microbiota (expert opinion). If prolonged antibiotic treatment is expected (i.e. more than 5–7 days), ADE should be performed as early as possible.
Challenges and Future Perspectives
As mentioned above, further research is needed to provide strong evidence in the field of ADE. The three ongoing studies are expected to provide information with regards to the safety of ADE in terms of mortality and superinfections. However, studies aimed at assessing the impact of ADE on the emergence of AMR are still required. These should be RCTs that measure the effect of ADE on the microbiota of the individual patients undergoing treatment and on the ICU ecosystem as a whole.
Conclusion
In this project experts in antimicrobial stewardship reviewed the existing literature and concluded that major questions remain regarding the clinical practice of ADE. We identified only one RCT on the topic and data are still lacking regarding adjusted patient outcomes and the effects of ADE on the emergence of antimicrobial resistance. Further research is needed to answer these questions.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Ines Lakbar, Alexis Tabah and Sharon Einav disclosed no conflict of interest. Marc Leone served as speaker for Aspen, Edwards Lifescience, MSD, Orion, Octapharma, Pfizer, and consultant for Aguettant and Amomed. Jan J. De Waele has consulted for Accelerate, Grifols, MSD and Pfizer (honorarium paid to institution). Ignacio Martin-Loeches received honorary fees as a member of the advisory boards of MSD and Gilead.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Footnotes
Digital Features
To view digital features for this article go to https://doi.org/10.6084/m9.figshare.12295583.
References
- 1.Tabah A, Bassetti M, Kollef MH, Zahar JR, Paiva JA, Timsit JF. Antimicrobial de-escalation in critically ill patients: a position statement from a task force of the European Society of Intensive Care Medicine (ESICM) and European Society of Clinical Microbiology and Infectious Diseases (ESCMID) Critically Ill. Intensive Care Med. 2019 doi: 10.1007/s00134-019-05866-w. [DOI] [PubMed] [Google Scholar]
- 2.Rhodes A, Evans LE, Alhazzani W, et al. Surviving Sepsis Campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43(3):304–377. doi: 10.1007/s00134-017-4683-6. [DOI] [PubMed] [Google Scholar]
- 3.Murri R, Taccari F, Palazzolo C, Fantoni M, Cauda R. IDSA did not endorse the Surviving Sepsis Campaign guidelines. Clin Infect Dis. 2018;66(11):1815–1816. doi: 10.1093/cid/cix1114. [DOI] [PubMed] [Google Scholar]
- 4.Leone M, Bechis C, Baumstarck K, et al. De-escalation versus continuation of empirical antimicrobial treatment in severe sepsis: a multicenter non-blinded randomized noninferiority trial. Intensive Care Med. 2014;40(10):1399–1408. doi: 10.1007/s00134-014-3411-8. [DOI] [PubMed] [Google Scholar]
- 5.Álvarez-Lerma F, Alvarez B, Luque P, et al. Empiric broad-spectrum antibiotic therapy of nosocomial pneumonia in the intensive care unit: a prospective observational study. Crit Care. 2006;10(3):1–11. doi: 10.1186/cc4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giantsou E, Liratzopoulos N, Efraimidou E, et al. De-escalation therapy rates are significantly higher by bronchoalveolar lavage than by tracheal aspirate. Intensive Care Med. 2007;33(9):1533–1540. doi: 10.1007/s00134-007-0619-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garnacho-Montero J, Gutiérrez-Pizarraya A, Escoresca-Ortega A, et al. De-escalation of empirical therapy is associated with lower mortality in patients with severe sepsis and septic shock. Intensive Care Med. 2014;40(1):32–40. doi: 10.1007/s00134-013-3077-7. [DOI] [PubMed] [Google Scholar]
- 8.Paskovaty A, Pastores SM, Gedrimaite Z, Kostelecky N, Riedel ER, Seo SK. Antimicrobial de-escalation in septic cancer patients: is it safe to back down? Intensive Care Med. 2015;41(11):2022–2023. doi: 10.1007/s00134-015-4016-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moraes RB, Guillén JAV, Zabaleta WJC, Borges FK. De-escalation, adequacy of antibiotic therapy and culture positivity in septic patients: an observational study. Rev Bras Ter Intensiva. 2016;28(3):315–322. doi: 10.5935/0103-507X.20160044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weiss E, Zahar JR, Garrouste-Orgeas M, et al. De-escalation of pivotal beta-lactam in ventilator-associated pneumonia does not impact outcome and marginally affects MDR acquisition. Intensive Care Med. 2016;42(12):2098–2100. doi: 10.1007/s00134-016-4448-7. [DOI] [PubMed] [Google Scholar]
- 11.De Bus L, Denys W, Catteeuw J, et al. Impact of de-escalation of beta-lactam antibiotics on the emergence of antibiotic resistance in ICU patients: a retrospective observational study. Intensive Care Med. 2016;42(6):1029–1039. doi: 10.1007/s00134-016-4301-z. [DOI] [PubMed] [Google Scholar]
- 12.Turza KC, Politano AD, Rosenberger LH, Riccio LM, McLeod M, Sawyer RG. De-escalation of antibiotics does not increase mortality in critically ill surgical patients. Surg Infect (Larchmt) 2016;17(1):48–52. doi: 10.1089/sur.2014.202. [DOI] [PubMed] [Google Scholar]
- 13.Trupka T, Fisher K, Micek ST, Juang P, Kollef MH. Enhanced antimicrobial de-escalation for pneumonia in mechanically ventilated patients: a cross-over study. Crit Care. 2017;21(1):1–8. doi: 10.1186/s13054-017-1772-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khan RA, Aziz Z. A retrospective study of antibiotic de-escalation in patients with ventilator-associated pneumonia in Malaysia. Int J Clin Pharm. 2017;39(4):906–912. doi: 10.1007/s11096-017-0499-2. [DOI] [PubMed] [Google Scholar]
- 15.Li H, Yang CH, Huang LO, et al. Antibiotics de-escalation in the treatment of ventilator-associated pneumonia in trauma patients: a retrospective study on propensity score matching method. Chin Med J (Engl) 2018;131(10):1151–1157. doi: 10.4103/0366-6999.231529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cowley MC, Ritchie DJ, Hampton N, Kollef MH, Micek ST. Outcomes associated with de-escalating therapy for methicillin-resistant Staphylococcus aureus in culture-negative nosocomial pneumonia. Chest. 2019;155(1):53–59. doi: 10.1016/j.chest.2018.10.014. [DOI] [PubMed] [Google Scholar]
- 17.Eachempati SR, Hydo LJ, Shou J, Barie PS. Does de-escalation of antibiotic therapy for ventilator-associated pneumonia affect the likelihood of recurrent pneumonia or mortality in critically ill surgical patients? J Trauma. 2009;66(5):1343–8. https://www.ncbi.nlm.nih.gov/pubmed/19430237 [DOI] [PubMed]
- 18.De Waele JJ, Ravyts M, Depuydt P, Blot SI, Decruyenaere J, Vogelaers D. De-escalation after empirical meropenem treatment in the intensive care unit: fiction or reality? J Crit Care. 2010;25(4):641–646. doi: 10.1016/j.jcrc.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 19.Morel J, Casoetto J, Jospé R, et al. De-escalation as part of a global strategy of empiric antibiotherapy management. A retrospective study in a medico-surgical intensive care unit. Crit Care. 2010;14(6):R225. https://ccforum.biomedcentral.com/articles/10.1186/cc9373 [DOI] [PMC free article] [PubMed]
- 20.Joung MK, Lee JA, Moon SY, et al. Impact of de-escalation therapy on clinical outcomes for intensive care unit-acquired pneumonia. Crit Care. 2011;15(2):R9. doi: 10.1186/cc10072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heenen S, Jacobs F, Vincent JL. Antibiotic strategies in severe nosocomial sepsis: why do we not de-escalate more often? Crit Care Med. 2012;40(5):1404–1409. doi: 10.1097/CCM.0b013e3182416ecf. [DOI] [PubMed] [Google Scholar]
- 22.Barraud D, Bollaert P-E, Gibot S, et al. Factors influencing the implementation of antibiotic de-escalation and impact of this strategy in critically ill patients. Crit Care. 2013;17(4):R140. https://ccforum.com/content/17/4/R140 [DOI] [PMC free article] [PubMed]
- 23.Knaak E, Cavalieri SJ, Elsasser GN, Preheim LC, Gonitzke A, Destache CJ. Does antibiotic de-escalation for nosocomial pneumonia impact intensive care unit length of stay? Infect Dis Clin Pract. 2013;21(3):172–176. [Google Scholar]
- 24.Mokart D, Slehofer G, Lambert J, et al. De-escalation of antimicrobial treatment in neutropenic patients with severe sepsis: results from an observational study. Intensive Care Med. 2014;40(1):41–49. doi: 10.1007/s00134-013-3148-9. [DOI] [PubMed] [Google Scholar]
- 25.Madaras-Kelly K, Jones M, Remington R, Hill N, Huttner B, Samore M. Development of an antibiotic spectrum score based on veterans affairs culture and susceptibility data for the purpose of measuring antibiotic de-escalation: a modified Delphi approach. Infect Control Hosp Epidemiol. 2014;35(9):1103–1113. doi: 10.1086/677633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weiss E, Zahar JR, Lesprit P, et al. Elaboration of a consensual definition of de-escalation allowing a ranking of β-lactams. Clin Microbiol Infect. 2015;21(7):649.e1–649.e10. doi: 10.1016/j.cmi.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 27.Singh N, Rogers P, Atwood CW, Wagener MM, Yu VL. Short-course empiric antibiotic therapy for patients with pulmonary infiltrates in the intensive care unit a proposed solution for indiscriminate antibiotic prescription. Am J Respir Crit Care Med. 2000;162. www.atsjournals.org [DOI] [PubMed]
- 28.Carlier M, Lipman J, De Waele JJ, Stove V, Roberts JA, Verstraete AG. A simulation study reveals lack of pharmacokinetic/pharmacodynamic target attainment in de-escalated antibiotic therapy in critically ill patients. Antimicrob Agents Chemother. 2015;59(8):4689–4694. doi: 10.1128/AAC.00409-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guilhaumou R, Benaboud S, Bennis Y, et al. Optimization of the treatment with beta-lactam antibiotics in critically ill patients—guidelines from the French Society of Pharmacology and Therapeutics (Société Française de Pharmacologie et Thérapeutique—SFPT) and the French Society of Anaesthesia and Intensive Care Medicine (Société Française d'Anesthésie et Réanimation-SFAR). Crit Care. 2019;23:104. [DOI] [PMC free article] [PubMed]
- 30.Leibovici L. Non-antibiotic treatment for bacterial infections: how to validate chance findings. Clin Microbiol Infect. 2009;15:298–301. [DOI] [PubMed]
- 31.Garnier M, Gallah S, Vimont S, et al. Multicentre randomised controlled trial to investigate usefulness of the rapid diagnostic βLACTA test performed directly on bacterial cell pellets from respiratory, urinary or blood samples for the early de-escalation of carbapenems in septic intensive care unit patients: the BLUE-CarbA protocol. BMJ Open. 2019;9(2):e024561. https://www.ncbi.nlm.nih.gov/pubmed/30782909 [DOI] [PMC free article] [PubMed]
- 32.López-Cortés LE, Rosso-Fernández C, Núñez-Núñez M, et al. Targeted simplification versus antipseudomonal broad-spectrum beta-lactams in patients with bloodstream infections due to Enterobacteriaceae (SIMPLIFY): a study protocol for a multicentre, open-label, phase III randomised, controlled, non-inferiority clinical trial. BMJ Open. 2017;7(6):e015439. https://www.ncbi.nlm.nih.gov/pubmed/28601833 [DOI] [PMC free article] [PubMed]
- 33.Mokart D. ClinicalTrials.gov identifier (NCT number): NCT03683329. https://clinicaltrials.gov/ct2/show/NCT03683329
- 34.Bhalodi AA, Van Engelen TSR, Virk HS, Wiersinga WJ. Impact of antimicrobial therapy on the gut microbiome. J Antimicrob Chemother. 2019;74:I6–15. doi: 10.1093/jac/dky530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Teshome BF, Vouri SM, Hampton N, Kollef MH, Micek ST. Duration of exposure to antipseudomonal β-lactam antibiotics in the critically ill and development of new resistance. Pharmacotherapy. 2019;39(3):261–270. doi: 10.1002/phar.2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lagier JC, Hugon P, Khelaifia S, Fournier PE, La Scola B, Raoult D. The rebirth of culture in microbiology through the example of culturomics to study human gut microbiota. Clin Microbiol Rev. 2015;28(1):237–264. doi: 10.1128/CMR.00014-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arnold C. Source code: putting metagenomics to the test in the clinic. Nat Med. 2017;23(6):645–648. doi: 10.1038/nm0617-645. [DOI] [PubMed] [Google Scholar]
- 38.Lanza VF, Baquero F, Martínez JL, et al. In-depth resistome analysis by targeted metagenomics. Microbiome. 2018;6(1):1–14. doi: 10.1186/s40168-017-0387-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coyne MJ, Zitomersky NL, McGuire AM, Earl AM, Comstock LE. Evidence of extensive DNA transfer between bacteroidales species within the human gut. MBio. 2014;5(3):1–12. doi: 10.1128/mBio.01305-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Willmann M, Peter S. Translational metagenomics and the human resistome: confronting the menace of the new millennium. J Mol Med. 2017;95(1):41–51. doi: 10.1007/s00109-016-1478-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Woerther PL, Lepeule R, Burdet C, Decousser JW, Ruppé É, Barbier F. Carbapenems and alternative β-lactams for the treatment of infections due to extended-spectrum β-lactamase-producing Enterobacteriaceae: what impact on intestinal colonisation resistance? Int J Antimicrob Agents. 2018;52(6):762–770. doi: 10.1016/j.ijantimicag.2018.08.026. [DOI] [PubMed] [Google Scholar]
- 42.Armand-Lefèvre L, Angebault C, Barbier F, et al. Emergence of imipenem-resistant gram-negative bacilli in intestinal flora of intensive care patients. Antimicrob Agents Chemother. 2013;57(3):1488–1495. doi: 10.1128/AAC.01823-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buelow E, Bello González TDJ, Fuentes S, et al. Comparative gut microbiota and resistome profiling of intensive care patients receiving selective digestive tract decontamination and healthy subjects. Microbiome. 2017;5(1):88. doi: 10.1186/s40168-017-0309-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Willmann M, Vehreschild MJGT, Biehl LM, et al. Distinct impact of antibiotics on the gut microbiome and resistome: a longitudinal multicenter cohort study. BMC Biol. 2019;17(1):1–18. doi: 10.1186/s12915-019-0692-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xiong W, Wang Y, Sun Y, et al. Antibiotic-mediated changes in the fecal microbiome of broiler chickens define the incidence of antibiotic resistance genes. Microbiome. 2018;6(1):1–11. doi: 10.1186/s40168-018-0419-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.