Figure 6. Liver glutathione-mediated biotransformation impacting in vivo transport of ICG4-GS-Au25.
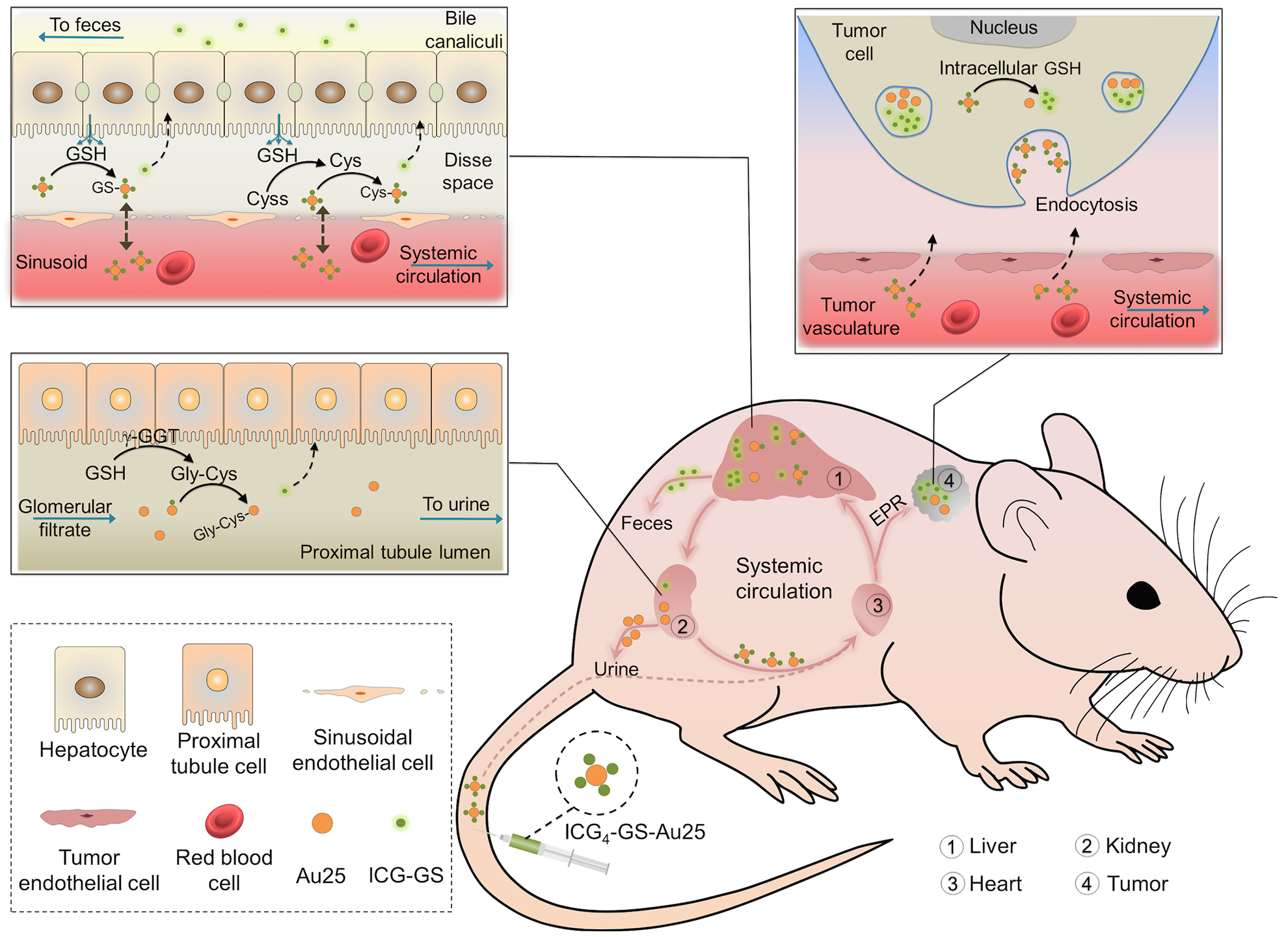
After intravenous administration, ICG4-GS-Au25 nanoclusters immediately bound to serum proteins. The protein-bound ICG4-GS-Au25 had an overall hydrodynamic size larger than kidney filtration threshold and thus was prevented from rapid renal elimination but was in part transported to the liver sinusoids, where the local high concentrations of glutathione and cysteine resulting from sinusoidal glutathione efflux displaced some or all of the ICG-GS from the surface of Au25, reducing the protein-binding affinity of ICG4-GS-Au25. The displaced ICG-GS was then taken up by hepatocytes and eliminated through the hepatobiliary pathway whereas the biotransformed ICG-GS-Au25 nanoclusters were back to the blood circulation and target tumor along with ICG4-GS-Au25 through EPR effect. When the biotransformed ICG-GS-Au25 nanoclusters circulated to the kidneys, those with low affinity to serum proteins passed the glomerular filtration and underwent additional surface modifications in kidney proximal tubules, where the left ICG-GS on Au25 nanoclusters were further displaced by cysteinylglycine, the extracellular metabolite of glutathione in proximal tubules. For those biotransformed ICG-GS-Au25 that still bound to serum proteins, they remained in the blood stream and continued to target the tumor. In the tumor, ICG4-GS-Au25 along with its biotransformed derivatives entered the tumor microenvironment and were internalized by the cells in tumor. The high concentration of intracellular glutathione then induced the dissociation of ICG-GS from Au25 within the cells and the tumor was lighted up for prolonged time. Some cell types such as Kupffer cells in liver and many stromal cells in tumor are omitted in this scheme for clarity.
